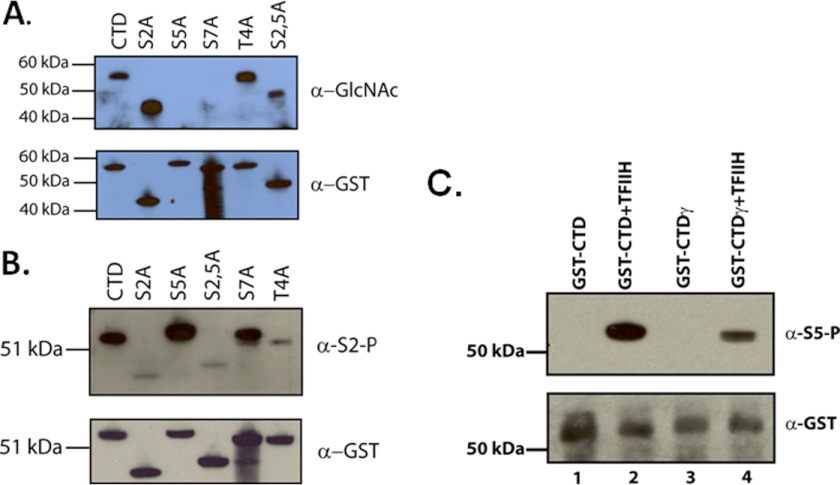
FIGURE 4.
OGT modifies the CTD at serine positions 5 and 7. A, GST-CTD or GST-CTD containing single alanine substitutions at serine positions 2 or 5, a threonine to alanine substitution at position 4, or a double substitution at positions 2 and 5 of the CTD (each containing ∼26 heptad repeats and each confirmed by sequencing). The lower panel is an example of the loading of the GST-CTD proteins in each lane as assayed by anti-GST antibody. The upper panel shows the results of incubations of the CTD substrates with rOGT and UDP-GlcNAc. The O-GlcNAc modification was detected using the 110.6 anti-O-GlcNAc antibody. B, control phosphorylation reactions with P-TEFb kinase and the GST-CTD or GST-mutant CTDs as described in A. Phosphorylation was detected by Western blot with the anti-phosphoserine 2 antibody H5. The lower panel indicates the loading of each protein as indicated in an anti-GST Western blot. C, O-GlcNAc-modified CTD was assayed for its efficiency as a substrate for TFIIH phosphorylation. GST-CTD or O-GlcNAc-modified GST-CTD (GST-CTDγ) was incubated with native TFIIH and ATP, and phosphorylation was assayed by Western blot with the serine 5-specific H14 antibody. The bottom panel illustrates the loading of equivalent amounts of GST-CTD as assayed by an anti-GST antibody. GST-CTDγ was produced with rOGT and UDP-GlcNAc.