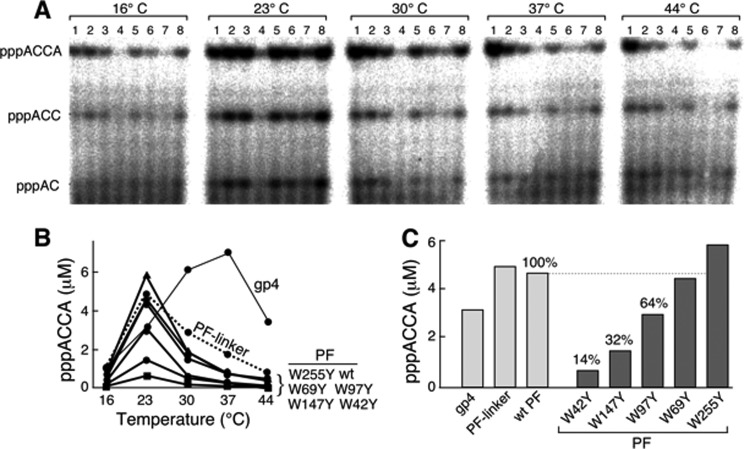
FIGURE 3.
Template-directed oligoribonucleotide synthesis by primases at different temperatures. A, the standard reaction for primer synthesis contained 0.2 μm gp4 or the indicated primase fragment, 4 μm template 1 containing 5′-TGGTC-3′ (Table 1), 1 mm ATP, and 1 mm [α-32P]CTP (0.1 mCi/ml) in buffer B. Wild-type primase fragment, primase fragments containing one tryptophan replaced with tyrosine, and primase fragment containing the linker between helicase and primase domains were used in these experiments. After incubation at 16, 23, 30, 37, or 44 °C for 30 min, the di-, tri-, and tetraribonucleotide products (indicated to the left) were separated on a 25% denaturing polyacrylamide gel. Lane 1, gp4; lane 2, primase fragment with a linker; lane 3, wild-type primase fragment (wt); lane 4, primase-W42Y; lane 5, primase-W69Y; lane 6, primase-W97Y; lane 7, primase-W147Y; lane 8, primase-W255Y. B, the amount of pppACCA formed in the reaction was measured and plotted against the reaction temperature for each protein. C, the amount of pppACCA synthesized at 23 °C by each protein is compared. The percentage was calculated by assigning a value of 100% for the wild-type primase fragment. Representative data from multiple experiments are shown.