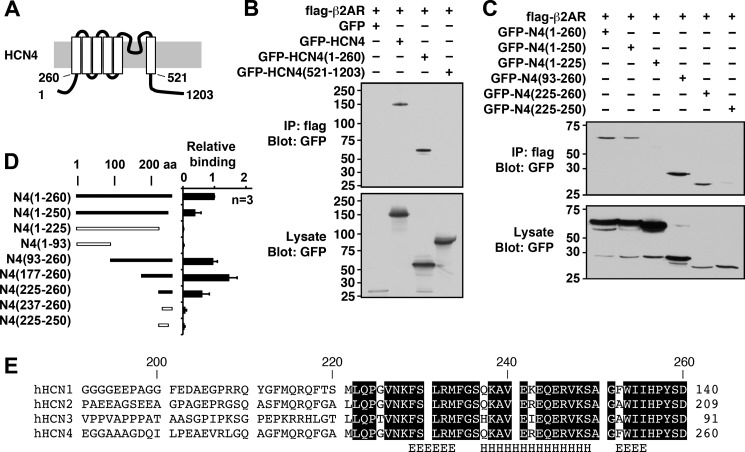
FIGURE 2.
Mapping analyses of the β2AR-binding site of the HCN4 channel. A, schematic diagram of the HCN4 channel showing the membrane topology and key residue numbers. B, immunoprecipitation (IP) using FLAG-β2AR and the GFP-tagged full-length HCN4 channel (amino acids (aa) 1–1203) and fragments containing the N terminus (amino acids 1–260) and the C terminus (amino acids 521–1203). C, immunoprecipitation using FLAG-β2AR and GFP-tagged N-terminal fragments. GFP-HCN4(225–260) was the shortest fragment demonstrating the interaction. D, summary of mapping analyses. Error bars show S.E. E, alignment of the β2AR-binding site for the HCN channel family. Conserved amino acids are boxed. The computer-predicted secondary structure for the helix (H) and the extended helix (E) are indicated. h, human.