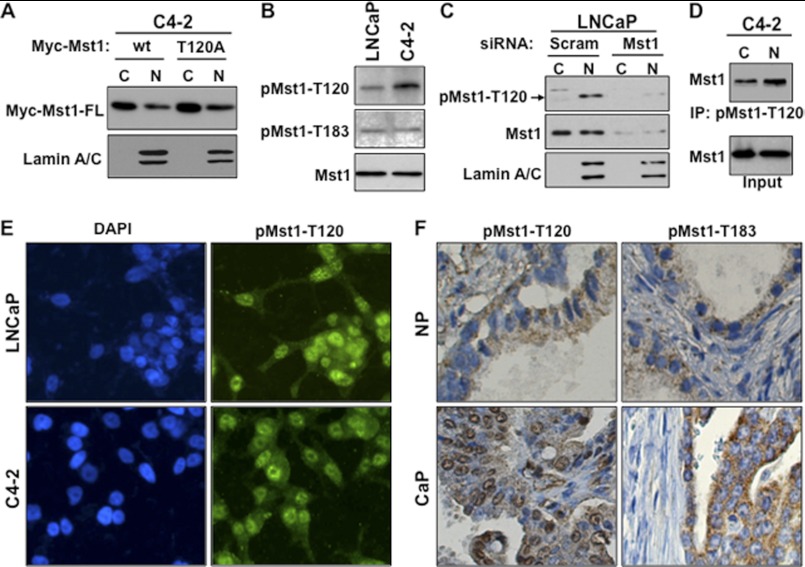
FIGURE 2.
Phospho-Thr-120 does not alter Mst1 nuclear localization and cleavage in prostate cancer cells. A, WB analysis of ectopically expressed Mst1-WT and Mst1-Thr-120 protein in cytoplasmic (C) and nuclear (N) fractions from C4-2 cells. Cells were transiently transfected with a Myc-tagged Mst1-WT and Mst1-Thr-120 plasmid. B, analysis of the total and phospho-Mst1-Thr-120 or -Thr-183 in total lysates of LNCaP or C4-2 cells by Western blot analysis. C, RNAi knockdown experiment. Cells were transfected with scramble or Mst1-specific siRNA, and levels of total or pMst1-Thr-120 were analyzed in cytoplasmic and nuclear fractions. 15 μg of total protein/lane was resolved by SDS-PAGE. D, IP with pMst1-Thr-120 antibody. Phospho-Mst1-Thr-120 protein was precipitated from nuclear and cytoplasmic fractions, respectively. 1 mg of total protein was used in the IP, and Western blot analyses were performed with antibody to the total Mst1 protein. E, IF staining of pMst1-Thr-120 in LNCaP and C4-2 PCa cells. Alexa Fluor 488-stained (green) pMst1-Thr-120 protein and DAPI-stained (blue) the nuclei (magnification ×20). F, IHC analysis of clinical samples from non-cancerous or normal prostate (n = 5) and cancerous prostate tissues (n = 15); magnification ×20. IHC was performed using pMst1–120 and pMst1-Thr-183 antibodies. Images are representative of multiple staining. NP, normal prostate; CaP, carcinoma of the prostate. Data are representative of multiple experiments.