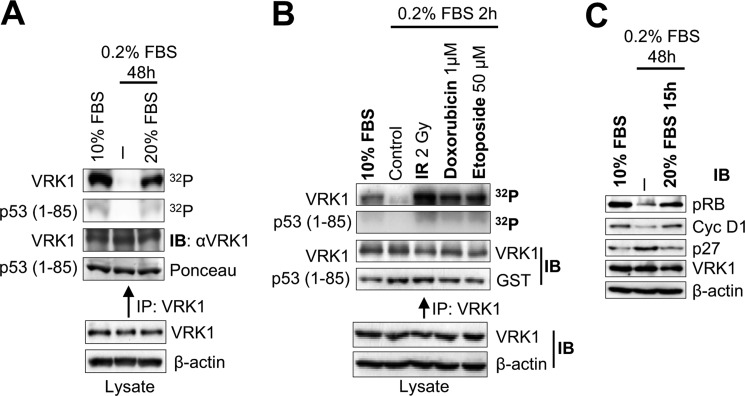
FIGURE 1.
VRK1 kinase activation in response to DNA damage. A, effect of serum withdrawal on the activity of VRK1. Endogenous VRK1 was immunoprecipitated from MCF7 cells either growing in 10% FBS (lane 1), serum-starved for 48 h (lane 2), or serum-starved for 48 h followed by 15 h in 10% FBS (lane 3). VRK1 activity was assayed in vitro for autophosphorylation and phosphorylation of GST-p53(1–85) fragment. B, kinase activity of endogenous VRK1 in response to DSBs. MCF7 cells were serum-starved and then treated with 2 Gy of ionizing radiation, 1 μm doxorubicin, or 50 μm etoposide. Endogenous VRK1 protein was immunoprecipitated and used in an in vitro kinase assay detecting its autophosphorylation and phosphorylation of GST-p53(1–85). C, detection of cell cycle markers by Western blot. MCF7 cells were grown in the presence of serum, serum-starved for 48 h, or serum-starved for 48 h followed by 15 h in serum. Extracts were blotted for phospho-Rb, cyclin D1, p27, VRK1, and actin. IP, immunoprecipitation; IB, immunoblot.