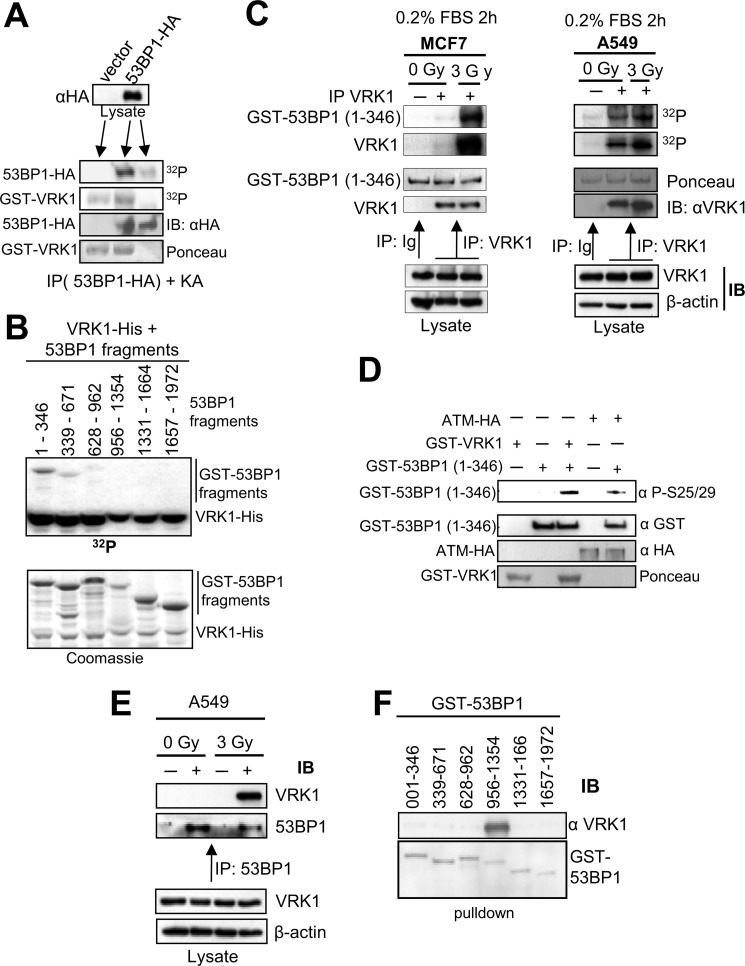
FIGURE 2.
Phosphorylation of 53BP1 by VRK1 and IR-induced interaction of VRK1 with 53BP1. A, full-length 53BP1-HA or empty vector was transfected in 293T cells and immunoprecipitated with anti-HA antibody. Immunoprecipitates were used as a substrate of GST-VRK1 in an in vitro kinase assay (25). B, GST-53BP1 fragments encoding different regions of 53BP1 as indicated were tested for phosphorylation by His-VRK1 in an in vitro kinase assay. The gel was exposed to x-ray films to detect phosphorylated proteins. C, starved MCF7 (left) or A549 (right) cells were left unirradiated or exposed to 3 Gy of γ rays. Endogenous VRK1 was immunoprecipitated 30 min after irradiation and assayed for autophosphorylation or phosphorylation of GST-53BP1(1–346) fragment in a kinase assay. Ig represents an immunoprecipitation with a nonspecific antibody (anti-HA). D, specific phosphorylation of 53BP1. In vitro phosphorylation of GST-53BP1(1–346) fragment was assayed either with GST-VRK1 (bacterially expressed) or ATM-HA (prepared by immunoprecipitation from 293T cells). The phosphorylation of 53BP1 was determined by Western blot with an anti-Ser-25/29 phosphospecific antibody (Cell Signaling Technology). E, induction of endogenous VRK1 and 53BP1 protein interaction by ionizing radiation. A549 cells were either left untreated or irradiated with 3 Gy. After 30 min, endogenous 53BP1 was immunoprecipitated using a polyclonal anti-53BP1 antibody. Control immunoprecipitations were performed with a polyclonal antibody (anti-FLAG). Endogenous VRK1 protein was only detected in the 53BP1 immunoprecipitate from irradiated cells. F, mapping the interaction region of 53BP1 with VRK1. Six GST-53BP1 fragments spanning the full-length 53BP1 protein as indicated were incubated with purified VRK1-His and subjected to GST pulldown (25, 74). Proteins were separated by SDS-PAGE followed by immunoblotting with anti-VRK1 antibody (1F6 mAb). IP, immunoprecipitation; IB, immunoblot.