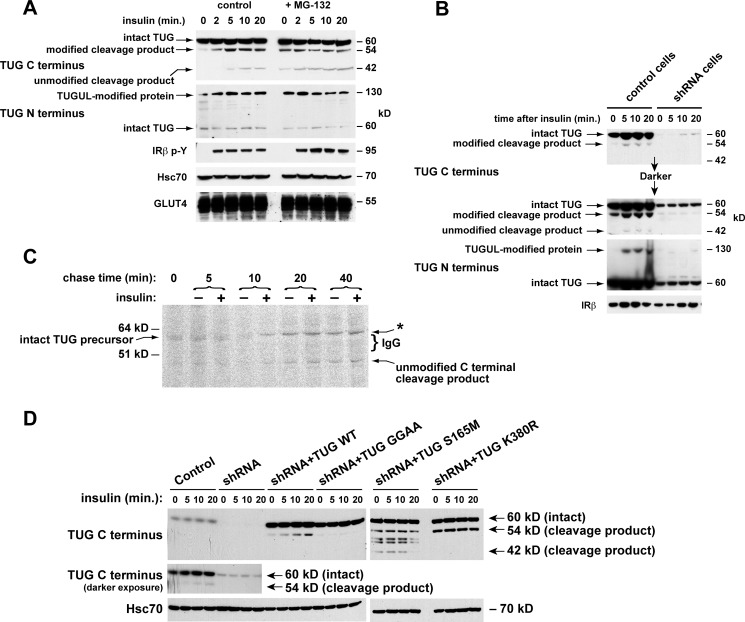
FIGURE 3.
Production of TUG derivatives by site-specific endoproteolytic cleavage. A, cells were treated or not with 10 μm MG-132 for 40 min and then with insulin, as indicated. After denaturing lysis, immunoblots were done as indicated. Controls show phosphorylation of the insulin receptor (IRβ p-Y) and equal loading (Hsc70 and GLUT4). B, control and shRNA cells were treated with insulin, lysed in denaturing conditions, and immunoblotted as indicated. IRβ, insulin receptor β-chain. C, cells were pulse-labeled with 35S-labeled Cys and Met, chased in non-radioactive amino acids and cycloheximide and in the presence or absence of insulin, and lysed at the indicated times using denaturing conditions. Immunoprecipitations were done with the TUG C terminus antibody, and eluted material was analyzed by SDS-PAGE and phosphorimaging. IgG was visualized by Coomassie staining (bracket) and probably altered migration of the 54-kDa C-terminal product observed in other experiments (asterisk). D, control cells, shRNA cells, and shRNA cells containing shRNA-resistant wild type or mutated TUG were treated with insulin as indicated. After denaturing lysis, immunoblots were done as indicated. Experiments were repeated at least twice with similar results.