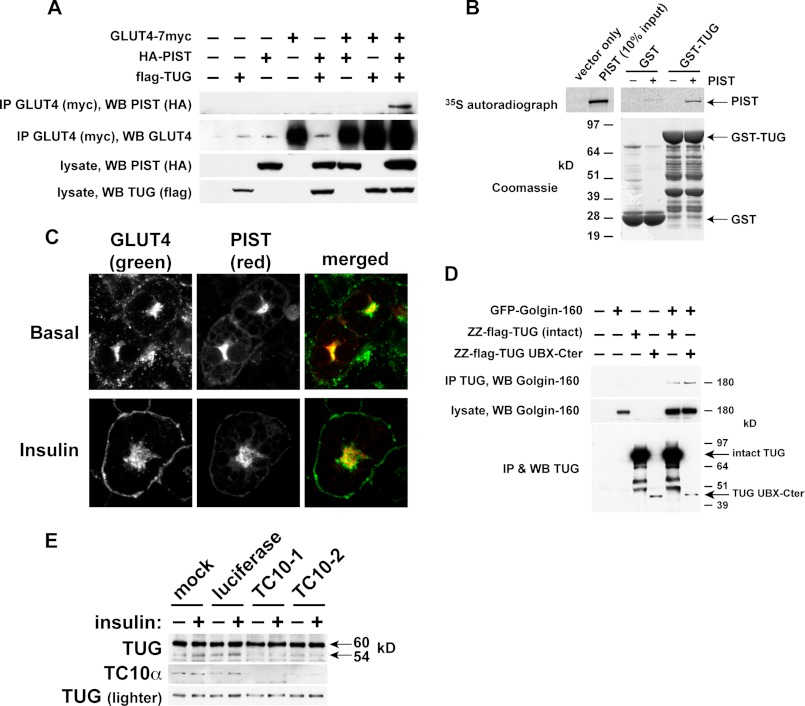
FIGURE 7.
Evidence that TUG cleavage separates GLUT4 from the Golgi matrix and requires TC10α. A, tagged forms of GLUT4, PIST, and TUG were transfected in 293T cells as indicated. GLUT4 was immunoprecipitated, and bound proteins were analyzed by SDS-PAGE and immunoblotting as indicated. B, PIST was translated in vitro in the presence of 35S-labeled Cys and Met and was incubated with recombinant GST-TUG or with GST alone. Bound proteins were analyzed by SDS-PAGE and autoradiography. C, basal and insulin-stimulated 3T3-L1 adipocytes stably expressing HA-PIST and GLUT4-GFP were imaged by confocal microscopy. Scale bar, 10 μm. D, GFP-tagged Golgin-160 and protein A (ZZ)-tagged TUG were transfected in 293T cells, and TUG was purified by binding to immobilized IgG. Eluates and lysates were immunoblotted as indicated. E, 3T3-L1 adipocytes were electroporated with synthetic siRNAs directed to TC10α or luciferase (as a control) or mock-electroporated. Cells were treated with or without insulin and then lysed using denaturing conditions and immunoblotted as indicated. All experiments were repeated at least twice with similar results.