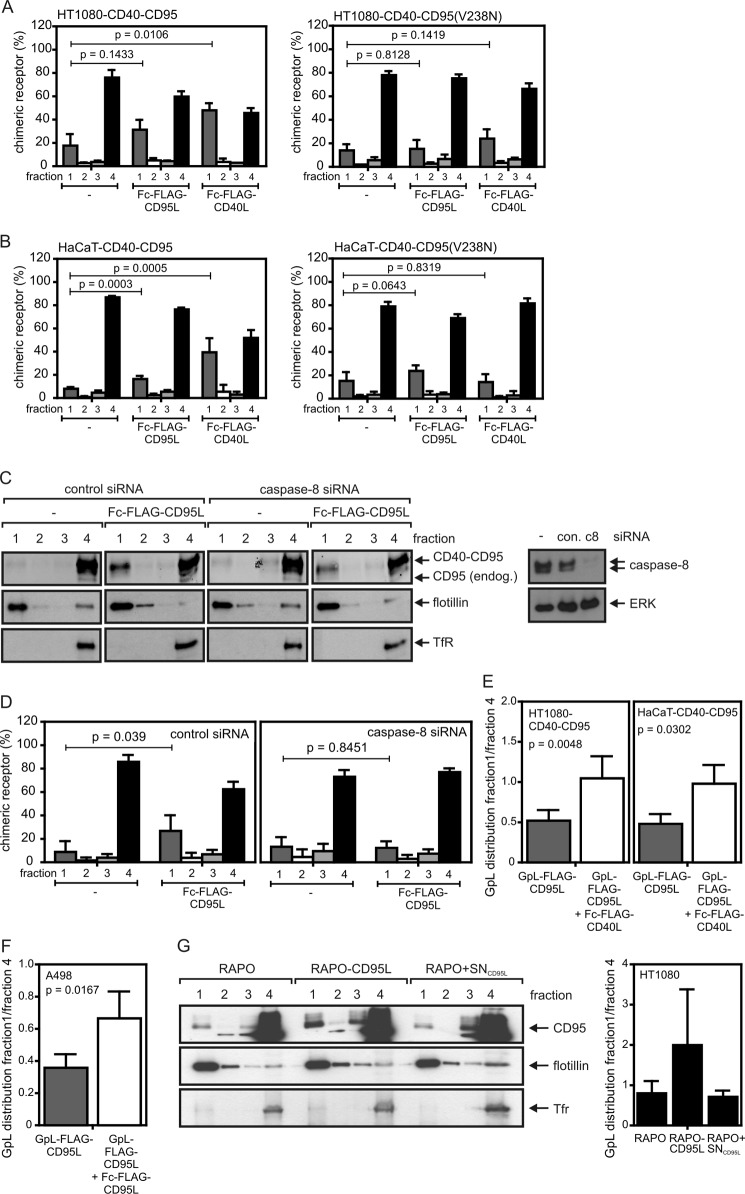
FIGURE 7.
Activation of CD95-associated signaling pathways triggers redistribution of unliganded CD40-CD95 chimeras and unliganded endogenous CD95 molecules into the detergent-insoluble compartment. A and B, the indicated HT1080 (A) and HaCaT (B) transfectants were stimulated for 2 h with 200 ng/ml Fc-FLAG-CD95L or 200 ng/ml Fc-FLAG-CD40L and subjected to lysis with Triton X-100 buffer and subsequent separation by sucrose density gradient centrifugation (fraction 1, insoluble membrane fraction with lipid rafts; fraction 4, soluble proteins). The intensity of the CD40-CD95 band of each fraction was quantified by LI-COR and normalized according to the total CD40-CD95 intensity of the corresponding gradient. Data shown are the average of three (A) and five (B) experiments. C and D, HaCaT-CD40-CD95 cells were transfected with control or caspase-8 specific siRNA. After 2 days, cells were stimulated for 2 h with 200 ng/ml Fc-FLAG-CD95L and were again analyzed by sucrose density gradient centrifugation and Western blotting for the distribution of the CD40-CD95 molecules. Western blot analysis of the sucrose density gradient fractions for CD40-CD95 distribution (left panel) and of total cell lysates for their caspase-8 content (right panel) are shown in C for one representative experiment. The averaged LI-COR quantification of CD40-CD95 distribution of five independent experiments is shown in D. Tfr, transferrin receptor. E, HT1080-CD40-CD95 and HaCaT-CD40-CD95 cells were incubated for 0.5 h with 25 ng/ml GpL-FLAG-CD95L to “label” a fraction of endogenous CD95 without activation of CD95-associated signaling pathways. Cells remained untreated or were then challenged with Fc-FLAG-CD40L for additional 2 h. Unbound ligands were removed by washing, and cells were lysed in Triton X-100 buffer and subjected to analysis by sucrose density gradient centrifugation. The distribution of the GpL-FLAG-CD95L was determined using a standard luciferase assay. Indicated is the mean ratio of relative light units of fraction 1 and fraction 4. F, a fraction of inactive endogenous CD95 of A498 cells was labeled by incubation with GpL-FLAG-CD95L (25 ng/ml) for 0.5 h. Remaining non-labeled CD95 molecules were then stimulated with Fc-FLAG-CD95L (200 ng/ml) for an additional 2 h. The distribution of GpL-FLAG-CD95L between the detergent soluble and insoluble compartment in stimulated and non-stimulated cells was then determined as in E. Shown is the average of three-five independent experiments (E and F). G, HT1080 cells were incubated for 30 min with GpL-FLAG-CD95L to mark inactive CD95 complexes and were then challenged for 4 h with RAPO cells, RAPO-CD95L cells, or RAPO cells supplemented with supernatant of RAPO-CD95L cells containing soluble CD95L (RAPO+SNCD95L). Triton X-100 lysates were separated by sucrose density gradient centrifugation, and the fractions obtained were analyzed by Western blotting (left panel) and luciferase activity assay (right panel) for the presence of CD95 and GpL-FLAG-CD95L. Analysis of luciferase activity shown is derived from four independent experiments.