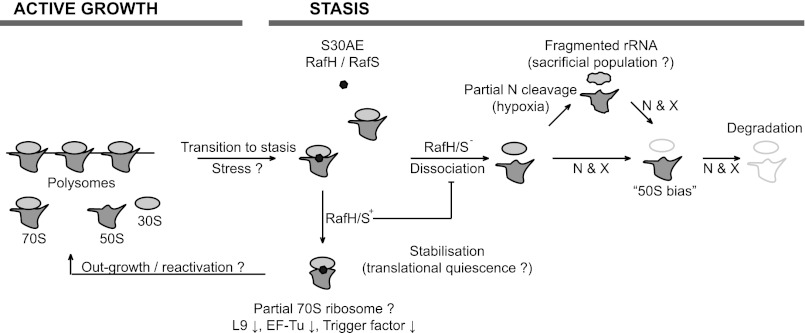
FIGURE 6.
Model of ribosomal stability during stasis. Active growth is characterized by the presence of actively translating ribosomes present either as 70S monomers or polysomes or free 50S and 30S subunits as they progress through the ribosomal cycle. Upon the cessation of growth (or exposure of stress), the S30AE domain proteins (RafH and RafS) become ribosome-attached and promote the associated form of the ribosome (70S), therefore leading to its stabilization and perhaps translational quiescence. We suggest that the S30AE-bound subpopulation of ribosomes is protected to allow the outgrowth of bacteria following stasis. Ribosomes that are not bound by S30AE proteins are more likely to expose the interface of the subunits, which serves as the substrate for endo- and exoribonucleases. Cleavage is likely to occur in a specific manner, leading to the fragmentation of rRNA (see Fig. 2). Although ribosomes may continue to exist with fragmented rRNA, perhaps as a “sacrificial population” to carry out the translation necessary to survive prolonged stasis, they eventually begin to disappear, leading to the complete degradation of the ribosome, breaking down first the 30S subunit leading to a 50S bias followed, finally, by the loss of the 50S subunit. S30AE, denotes RafH (MSMEG_3935) or RafS (MSMEG_1878); N, specific endonucleases; X, specific exonucleases; L9, large ribosomal subunit protein L9; EF-Tu, elongation factor Tu.