Figure 3. Effect of oxidative and nitrosative modifications on the calcium dependence of the cardiac ryanodine receptor (RyR2).
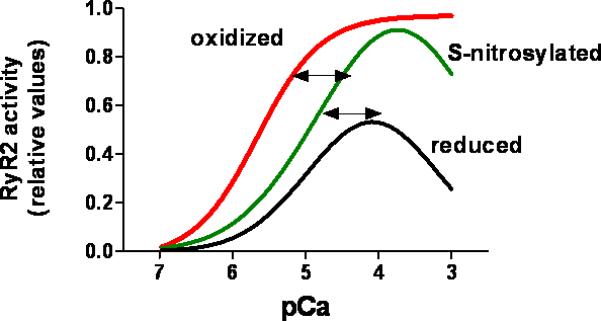
RyR is constitutively and reversibly S-nitrosylated, which increases RyR2 activity under normal conditions. Hyper- and hypo-nitrosylation may contribute to disease. Cysteine oxidation, likely a pathological modification, progressively increases RyR2 responsiveness to activating cytosolic Ca2+. Oxidative activation may be irreversible. The values for RyR2 activity are relative and were obtained from the literature as open probability and 3[H]ryanodine binding.
