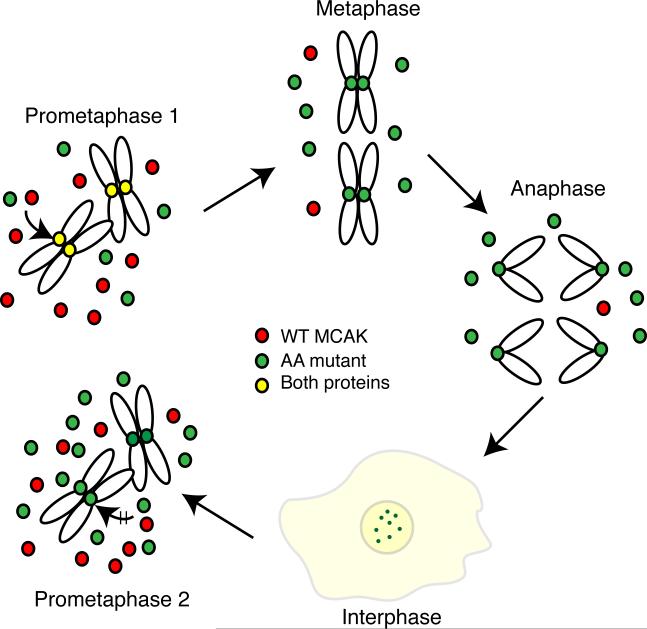
Fig. 9. Model showing the effects of the S628A/S629A mutations on MCAK function.
After the induction of the AA mutant, cells enter the first mitosis (prometaphase 1) with naive endogenous WT (red) and ectopic AA mutant (green) proteins that require Aurora B kinase phosphorylation before they can bind to centromeres (yellow to show the presence of both endogenous and AA mutant). Once cells attain a stable metaphase configuration, the WT protein is degraded because it is phosphorylated on residue 628 leaving only the AA mutant protein on centromeres. The AA mutant protein persists through anaphase, telophase, and into the next cell cycle where it, but not the newly synthesized WT protein, remains bound to centromeres. Diffuse staining is also seen for both WT and mutant proteins (light yellow color). As cells enter the next mitosis (prometaphase 2), the amount of mutant protein is higher relative to the WT protein because of its failure to be degraded during the previous mitosis. We propose that toxicity results from the increased total amount of MCAK as well as the centromeric localization of modified AA mutant protein carried over from the previous cell cycle that may disrupt the binding of newly synthesized naive MCAK and interfere with the phosphorylations that normally regulate MCAK activity during mitosis.