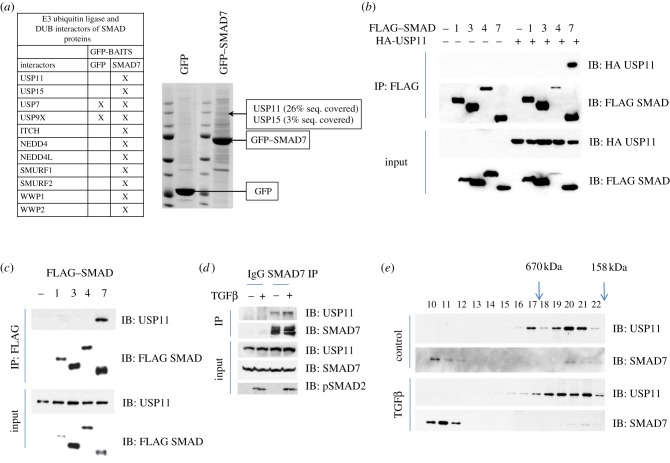
Figure 1.
Identification and characterization of USP11 as an interactor of SMAD7. (a) Representative Coomassie-stained gels showing anti-GFP IPs from HEK293 extracts expressing GFP-alone or GFP-SMAD7. The interacting proteins were excised as 2 mm gel pieces, digested with trypsin and identified by mass spectrometry. The gel piece from which USP11 was identified is indicated. A summary table of various Smad-interacting E3 ubiquitin ligases and DUBs identified by mass spectrometry is included. The sequence coverage of USP11 and USP15 in GFP-SMAD7 IPs is indicated. (b) HEK293 cells were co-transfected transiently with HA-USP11 and FLAG–SMADs. FLAG IPs and lysate inputs were immunoblotted with FLAG and HA antibodies as indicated. (c) HEK293 cells were transiently transfected with FLAG–SMADs only. FLAG IPs and lysate inputs were immunoblotted with FLAG and endogenous USP11 antibodies. (d) Lysates from HEK293 cells treated with vehicle or TGFβ (50 pM 45 min) were immunoprecipitated using pre-immune IgG or a SMAD7 antibody covalently bound to Dynabeads (Invitrogen). IPs and lysate inputs were immunoblotted with endogenous USP11, SMAD7 and phospho-SMAD2 antibodies. (e) Extracts from HaCaT cells starved for 4 h and stimulated with or without 50 pM TGFβ for 1 h were separated by size-exclusion gel chromatography. The collected fractions were immunoblotted with anti-USP11 and anti-SMAD7 antibodies.