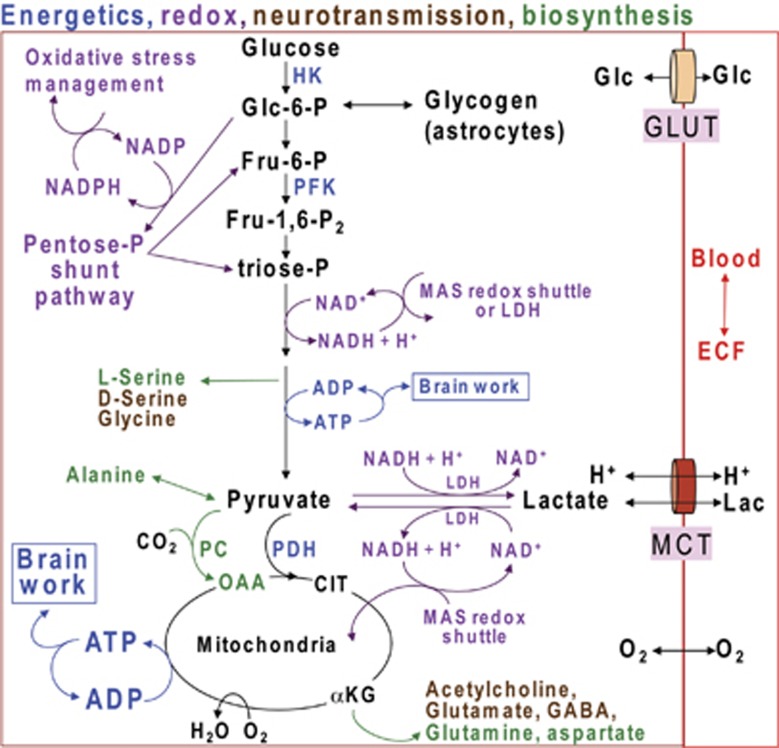
Figure 1.
Multifunctional roles of glucose metabolism. Color coding denotes different functional roles of pathways of glucose metabolism. Glucose (Glc) and lactate (Lac) plus H+ are transported into brain cells from blood or extracellular fluid (ECF) by equilibrative transporters, GLUTs and MCTs (monocarboxylic acid transporters), respectively, whereas oxygen diffuses into brain cells. Energetics (blue) involves ATP production by the glycolytic (glucose to pyruvate; Fru=fructose) and oxidative (pyruvate to CO2 + H2O) pathways. Glycolytic rate is modulated by regulation of hexokinase (HK), phosphofructokinase (PFK), and other enzymes. Glucose is stored as glycogen, mainly in astrocytes. Pyruvate enters the oxidative pathway of the mitochondrial tricarboxylic acid cycle, with formation of 3 CO2 and regeneration of oxaloacetate (OAA). Neurotransmitters and neuromodulators (brown) are synthesized through the glycolytic and oxidative pathways. Other biosynthetic pathways produce amino acids (green) and sugars (not shown) used to synthesize complex carbohydrates for glycoproteins and glycolipids. Net synthesis of a ‘new' four- or five-carbon compound (aspartate, glutamate, glutamine, GABA) requires pyruvate carboxylase (PC), which is only located in astrocytes. CO2 fixation by PC converts pyruvate to OAA. OAA is transaminated to form aspartate or it condenses with acetyl CoA derived from a second pyruvate molecule by the action of pyruvate dehydrogenase (PDH) to form citrate (CIT). Decarboxylation of this ‘new' six-carbon compound forms α-ketoglutarate (αKG) that can be converted to a new molecule of glutamate, glutamine, and GABA. These compounds can also incorporate label from labeled glucose in neurons and astrocytes by means of reversible exchange reactions, but their net synthesis requires the astrocytic PC reaction. Acetylcholine is also derived from glucose through citrate in neurons. Entry of glucose-6-P into the pentose phosphate shunt pathway (purple) results in oxidative decarboxylation of carbon one of glucose and generates NADPH, which is used to detoxify reactive species that can cause oxidative stress. The nonoxidative branch of pentose shunt produces fructose-6-phosphate (Fru-6-P) and triose-P that reenter the glycolytic pathway; nucleotide precursors are also generated by the pentose shunt pathway. NAD+ is required for glycolysis, and it is regenerated from NADH by the malate–aspartate shuttle (MAS) or lactate dehydrogenase (LDH) (purple). The MAS redox shuttle transfers reducing equivalents from the cytosol to the mitochondria and is required to generate pyruvate for oxidation by the tricarboxylic acid cycle; it is also required for oxidative metabolism of lactate. Regeneration of NAD+ by LDH removes pyruvate from the oxidative pathway.