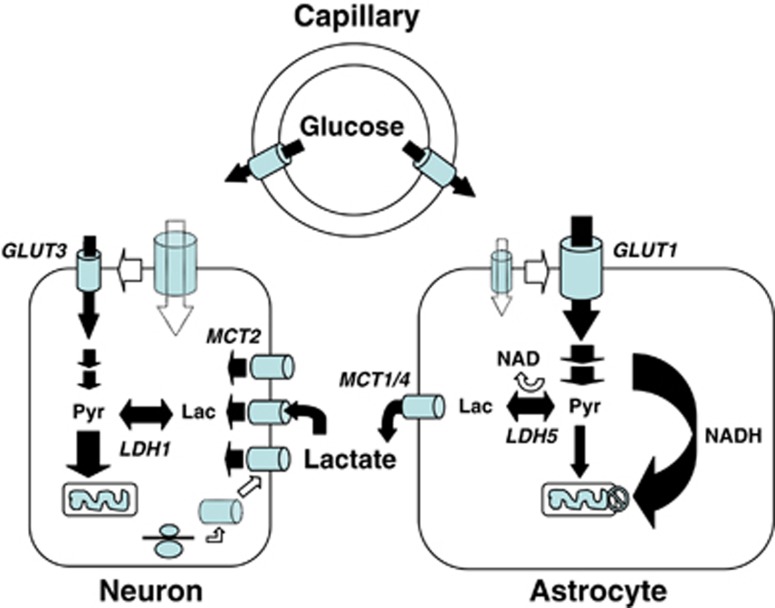
Figure 5.
Importance of monocarboxylate transporters and the regulation of their expression/localization in lactate shuttling between astrocytes and neurons. Several unique features of astrocytes and neurons have been uncovered that suggest a partial metabolic compartmentalization and the existence of a preferential lactate transfer between the two cell types. Thus, exposure of astrocytes to glutamate was shown to directly enhance glucose transport in parallel with increased glucose utilization. Conversion of pyruvate into lactate in astrocytes is facilitated by key characteristics. Astrocytes lack a mitochondrial aspartate/glutamate carrier that reduces their capacity to transfer reducing equivalent as nicotinamide adenine dinucleotide (NADH) by the malate/aspartate shuttle in the mitochondria and regenerate NAD. To maintain the glycolytic flux, cytosolic NADH is rather converted to NAD through the reaction catalyzed by the lactate dehydrogenase isoform LDH5, preferentially expressed in astrocytes. Lactate formed is then released in the extracellular space via the high-capacity monocarboxylate transporters, MCT1 and MCT4. In contrast to astrocytes, glucose uptake in neurons is reduced by glutamate. Moreover, ascorbic acid that is shuttled from astrocytes to neurons also contributes to reduce glucose uptake in neurons. In parallel, ascorbic acid enhances lactate uptake by neurons, and lactate conversion to pyruvate is facilitated by the preferential expression of the lactate dehydrogenase isoform, LDH1. Expression of the neuronal monocarboxylate transporter, MCT2, can be enhanced through an increase in protein synthesis by various stimuli, which might contribute to long-term adaptation of energy supply to demand. GLUT, glucose transporter; Lac, lactate; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; Pyr, pyruvate. Taken from Pellerin (2008).