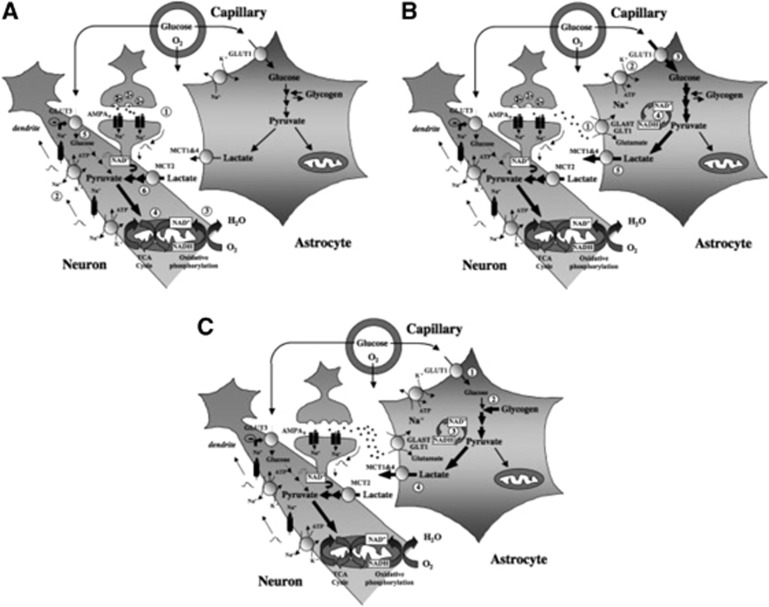
Figure 6.
Contribution of glucose- and glycogen-derived lactate to support different phases of neuronal activation. (A) Early phase. Activation of glutamatergic afferents leads to synaptic release of glutamate, AMPA receptor (AMPAR) activation, and generation of an excitatory postsynaptic potential (EPSP) caused by Na+ entry within the postsynaptic spine (1). Depolarization propagates to the dendrite and causes opening of voltage-sensitive Na+ channels, leading to further Na+ entry. Reestablishment of ion gradients is accomplished by the Na+/K+ ATPase (2), which creates considerable energy expenditure. As a consequence, oxidative phosphorylation is activated (3) and mitochondrial nicotinamide adenine dinucleotide (NADH) levels first decrease (Kasischke et al, 2004). Then, enhanced tricarboxylic acid (TCA) cycle activity will ensue (4) to supply NADH for oxidative phosphorylation and support ATP production. As pyruvate utilization in the TCA cycle increases and its cytoplasmic levels decrease, the conditions become favorable for both enhanced glucose and lactate use. Surprisingly, activation of AMPA receptors and coupled Na+ entry lead to a reduction in glucose uptake and utilization in neurons (5), thus further favoring lactate utilization as preferential oxidative substrate (6). This would cause a transient drop in extracellular lactate levels as measured in vivo. (B) Late phase. Glutamate released in the synaptic cleft is taken up by astrocytes to be recycled via the specific glutamate transporters GLAST and GLT1 (1). A large Na+ influx caused by glutamate uptake takes place and activates the Na+/K+ ATPase (2), glucose transport (3) and (4) glucose utilization in astrocytes. The enhancement of aerobic glycolysis in astrocytes first causes a large increase in cytosolic NADH that normalizes with the conversion of pyruvate into lactate and its release via monocarboxylate transporters expressed on astrocytes (mainly MCT1 and 4) (5). Such a lactate release following glutamatergic activation corresponds to the increase in extracellular lactate levels measured in vivo. Lactate produced by astrocytes during this later phase of activation not only replenishes the extracellular pool but also could help sustain neuronal energy needs as activation persists. Metabolic events occurring in the early and late phases described above constitute the so-called astrocyte–neuron lactate shuttle and its importance grows with the degree of glutamatergic activation. Such a view is supported by a series of experiments conducted in vivo. (C) Intense and prolonged stimulation. On strong and long-lasting stimulation that occurs in certain conditions, glucose utilization becomes very important, in part due to intense glutamate reuptake in astrocytes, that extracellular glucose levels are insufficient to sustain such uptake (1). In such a situation, glycogen present in the astrocyte is mobilized to provide the necessary glycosyl units (2) as previously demonstrated in vivo. Glycolysis is the predominant pathway (3) and lactate is produced (4) to maintain the high glycolytic rate. Resynthesis of glycogen will cause additional glucose uptake that might contribute to create a mismatch between glucose utilization and oxygen consumption, a phenomenon known as ‘uncoupling.' Taken from Pellerin et al (2007).