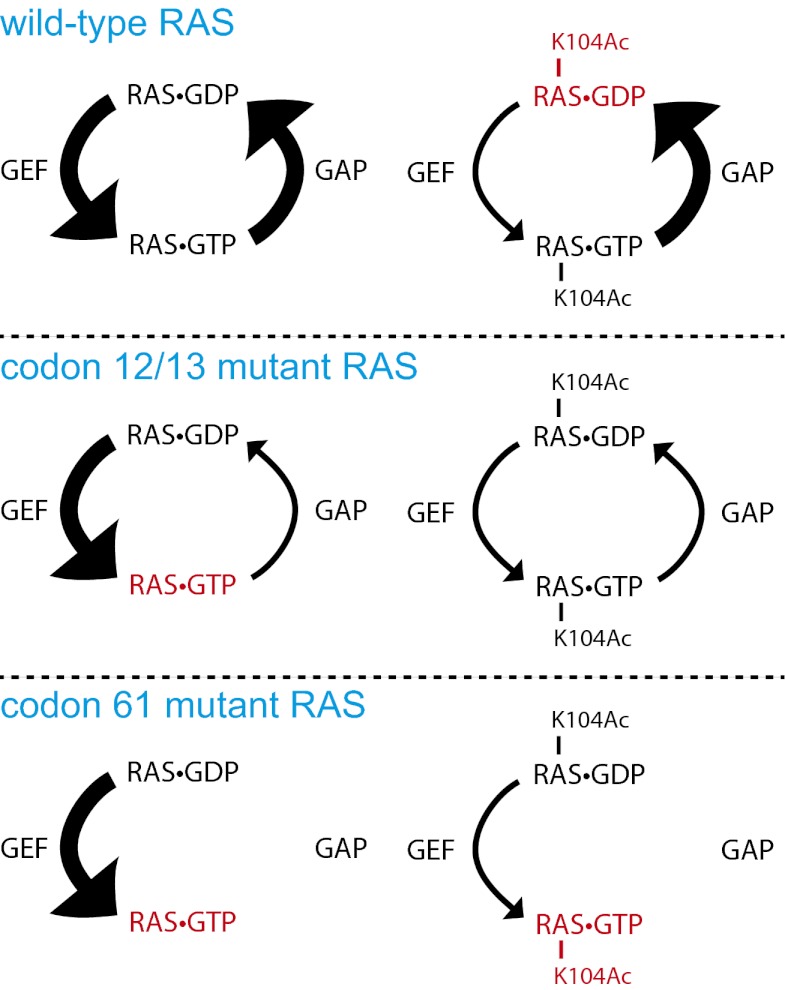
Fig. 5.
Model for the effect of K104 acetylation on wild-type and mutant RAS. When RAS is wild-type and unacetylated, its nucleotide-binding state is largely controlled by GAP and GEF, creating an equilibrium between RAS⋅GTP and RAS⋅GDP. Acetylation of K104 causes a shift in the equilibrium toward RAS⋅GDP because of insensitivity to GEF. When RAS is mutationally activated at codon 12 or 13, the equilibrium is shifted toward RAS⋅GTP because of insensitivity to GAP. This equilibrium shift can be counteracted by K104 acetylation, which suppresses GEF-induced nucleotide exchange. When RAS is mutationally activated at codon 61, the equilibrium is shifted toward RAS⋅GTP because of the loss of intrinsic GTPase activity. This equilibrium shift cannot be affected by K104 acetylation. Red lettering indicates the nucleotide state of RAS that is favored under each circumstance.