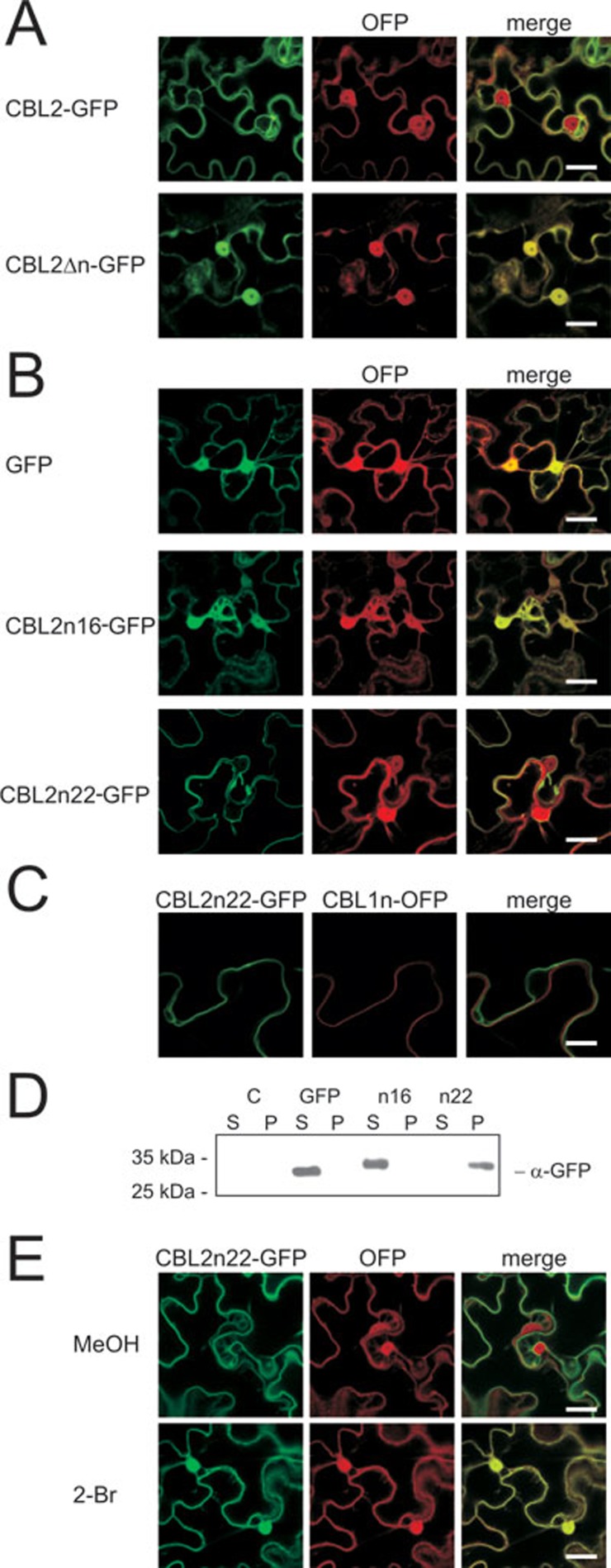
Figure 2.
The N-terminal CBL2 fragment, harboring three cysteine residues, is sufficient for efficient targeting to the vacuolar membrane. (A) The first 15-aa from the CBL2 N-terminus were removed from the protein (CBL2Δn). A GFP fusion protein was transiently expressed in N. benthamiana leaves for 2 days, together with a soluble cytosolic OFP marker protein. Bars in the merged pictures represent 20 μm. (B) Different fragments of the CBL2 N-terminus harboring either 16-aa (with the first two cysteine residues) or 22-aa (with three cysteine residues) were fused to the N-terminus of GFP. The various GFP fusion proteins were transiently expressed in N. benthamiana leaves for 2 days together with the soluble cytosolic marker protein OFP, and microscopically analyzed. Bars in the merged pictures represent 20 μm. (C) CBL2n22-GFP does not associate with the plasma membrane. CBL2n22-GFP and the plasma membrane associated CBL1n-OFP marker protein were transiently expressed in N. benthamiana leaves for 2 days. Bars represent 10 μm. (D) Different fragments of the CBL2 N-terminus harboring either 12-aa (with the first two cysteine residues) or 22-aa (with all three cysteine residues) were fused to the N-terminus of GFP. Wild-type GFP was used as a control. Proteins were transiently expressed in N. benthamiana leaves for 2 days. Native proteins were extracted and sub-cellularly fractionated by a 1 h 100 000× g centrifugation step. The soluble (S) and pellet (P) protein fractions were analyzed by western blotting as described in the Materials and Methods section. Mock-infiltrated leaves (expressing a HA tagged protein) were used to prepare a control sample (C). (E) Membrane association of CBL2n22-GFP is affected by 2-Bromopalmitate. CBL2n22-GFP was transiently expressed in N. benthamiana leaves for 2 days together with the soluble cytosolic marker protein OFP. One day after the leaf infiltration, samples were taken from the leaves and incubated in tap water with either 0.05% MeOH and 0.005% Tween (MeOH control) or 50 μM 2-Bromopalmitate and 0.005% Tween (2-Br), incubated for 16 h and microscopically analyzed. Bars represent 20 μm.