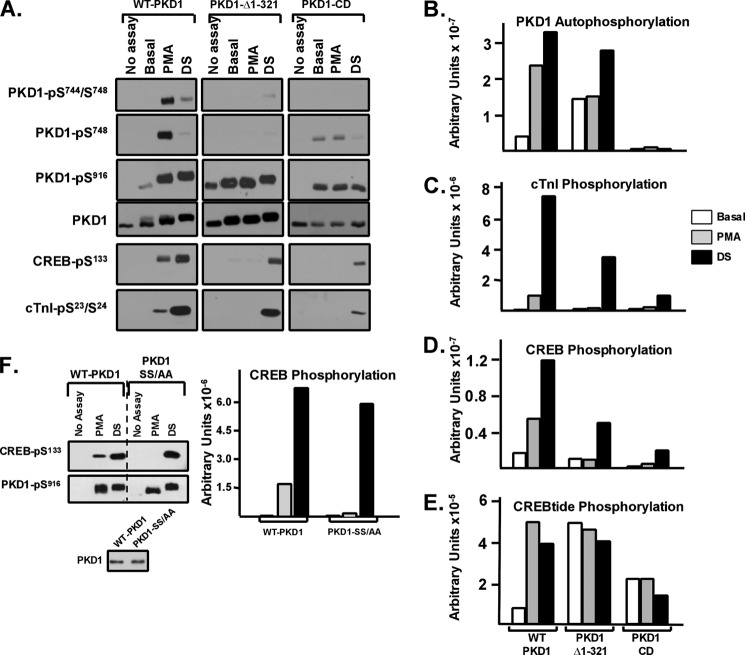
FIGURE 2.
PKD1-Δ1–321 and PKD1-CD are CREBtide kinases that autophosphorylate at Ser916 but do not effectively autophosphorylate their activation loop or trans phosphorylate CREB or cTnI. WT-PKD1, PKD1-Δ1–321, and PKD1-CD were immunoprecipitated from HEK293 cells and used in IVKAs in buffers without or with PMA or dextran sulfate (with CREB, cTnI, or CREBtide as substrate) as described under “Experimental Procedures.” A, immunoblotting for PKD1 activation loop and Ser916 autophosphorylation, PKD1 protein (to show similar recovery and loading of each enzyme), CREB-Ser133 phosphorylation, and cTnI-Ser23/Ser24 phosphorylation. B, C, and D, 32P incorporation into PKD1, cTnI, and CREB quantified by PhosphorImager. All results are from a single experiment, with similar results obtained in two separate experiments. E, PKD1-dependent CREBtide phosphorylation was examined in triplicate as described under “Experimental Procedures”; data are from a single experiment, with identical results obtained in two separate experiments. F, WT-PKD1 and PKD1-S744A/S748A (PKD1-SS/AA) were immunoprecipitated from HEK293 cells, and IVKAs were performed in buffers without or with PMA or DS with CREB as substrate. Immunoblotting was for PKD1-Ser916 and CREB-Ser133 phosphorylation or PKD1 protein (to show equal recovery of WT-PKD1 and PKD1-SS/AA), with 32P incorporation into CREB quantified by PhosphorImager. Data are from a single experiment that was replicated in two separate experiments.