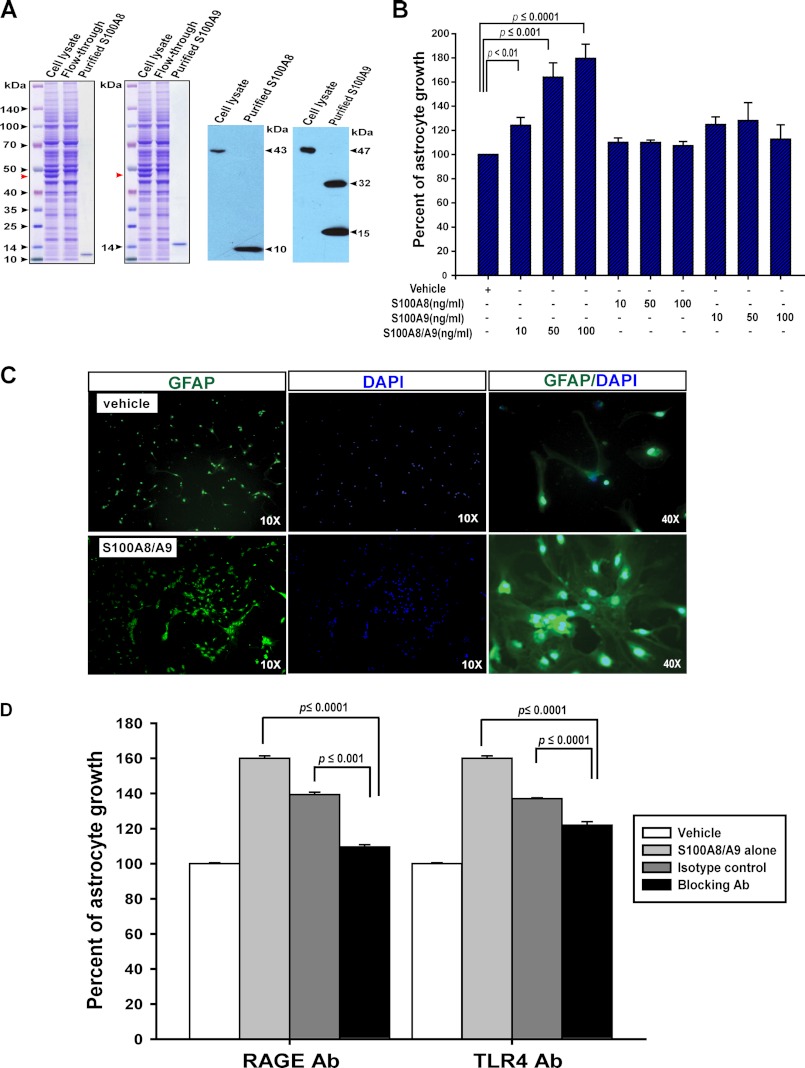
FIGURE 6.
S100A8-S100A9 complex promotes astrocyte growth in vitro partially through the TLR4 and RAGE receptors. A, purification of mouse S100A8 and S100A9 proteins from transiently transfected HEK293T cells. Left panels, Coomassie Blue-stained SDS-polyacrylamide gels show purification of mS100A8 and mS100A9 proteins using the HaloTag purification system. Cell lysate represents the total protein extracts from transiently transfected HEK292T cells; flow-through represents the unbound lysate after an overnight incubation with HaloLink resin, and purified proteins are the tag-free mS100A8 and mS100A9 proteins after HaloTag-TEV protease cleavage of the HaloLink resin. Red arrowheads indicated the mS100A8-Halo or mS100A9-Halo overexpressed in HEK293T cells. Right panels, Western blot analysis of S100A8 and S100A9 confirms the identities of purified proteins. Note: minor fraction of mS100A9 was purified as homodimers, similar to purified hS100A9 in other reports. B, C, and D, cortices were isolated from wild-type P1 neonates and dissociated. Cells were cultured in vitro to promote astrocyte growth. Confluent astrocytes were split and seeded in triplicate in 96-well plates. Astrocytes were cultured for 96 h before analyses. B, cell viability was analyzed by CellTiter Glo assay. Percentages of astrocyte growth in response to S100A8 alone, S100A9 alone, or S100A8-S100A9 complex were quantified relative to vehicle control, which is arbitrarily set at 100%. Results are presented as average + S.D. (n = 5). C, representative images of GFAP staining (green) in wild-type astrocytes cultured in the presence of 100 ng/ml S100A8-S100A9 complex or vehicle. The cell nuclei were counterstained with DAPI (blue). D, astrocyte cultures were set up as described under “Materials and Methods.” Cell viability was analyzed by CellTiter Glo assay. Percentages of astrocyte growth in response to S100A8-S100A9 alone or S100A8-S100A9 with different isotype controls or blocking antibodies were quantified relative to vehicle control, which is arbitrarily set at 100%. Results are presented as average + S.D. (n = 3).