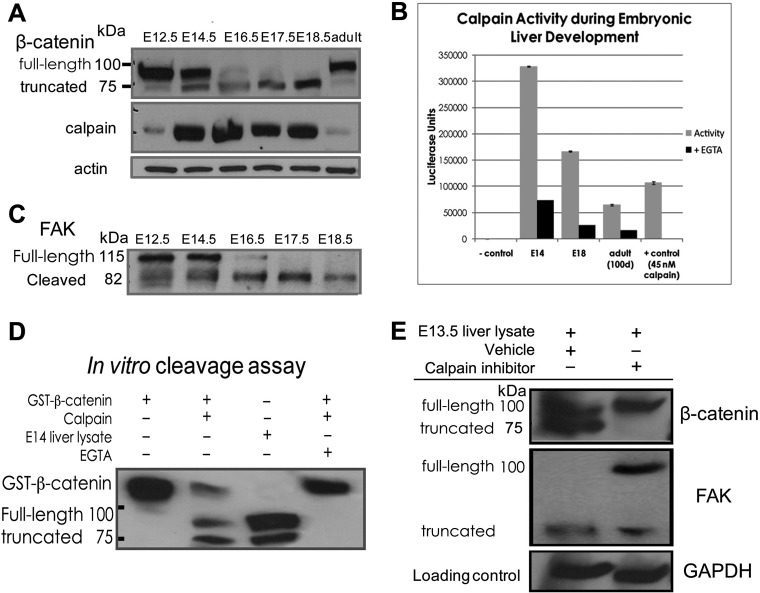
FIGURE 3.
Calpain cleavage of β-catenin is responsible for production of the truncated β-catenin species. A, immunoblot analysis indicates that calpain is expressed in developing liver and that its expression level is highest at times when β-catenin cleavage is observed. B, calpain-GLO activity assay detects active calpain during liver development, with activity highest at E14.5, when significant β-catenin cleavage appears, and persisting at E18.5 when the truncated β-catenin is the predominant form present. Calpain activity is lowest but still present in adult samples when minimal β-catenin cleavage is observed. No calpain activity is seen in samples lacking the addition of liver lysates or purified calpain (far left), and positive control for calpain (45 nm purified enzyme added) is positive for calpain activity (far right). In each case the addition of EGTA, a known calpain inhibitor, results in significant quenching of calpain activity. C, an immunoblot for the known calpain substrate focal adhesion kinase (FAK) shows a cleavage pattern paralleling that of β-catenin and provides further evidence that calpain is active in developing liver. (D). In vitro protease assay combining purified calpain and recombinant GST-tagged β-catenin produces a β-catenin cleavage pattern identical to that seen in developing liver. This cleavage is inhibited by EGTA, a known inhibitor of calpain activity. E, livers from embryos of timed pregnant mice at 13.5 days post-coitus injected intraperitoneally with the calpain inhibitor MDL28170 (12.5 mg/kg) show a lack of truncated β-catenin production relative to vehicle-treated animals. Immunoblot performed in parallel for focal adhesion kinase shows that the calpain-mediated cleavage of focal adhesion kinase is inhibited as well.