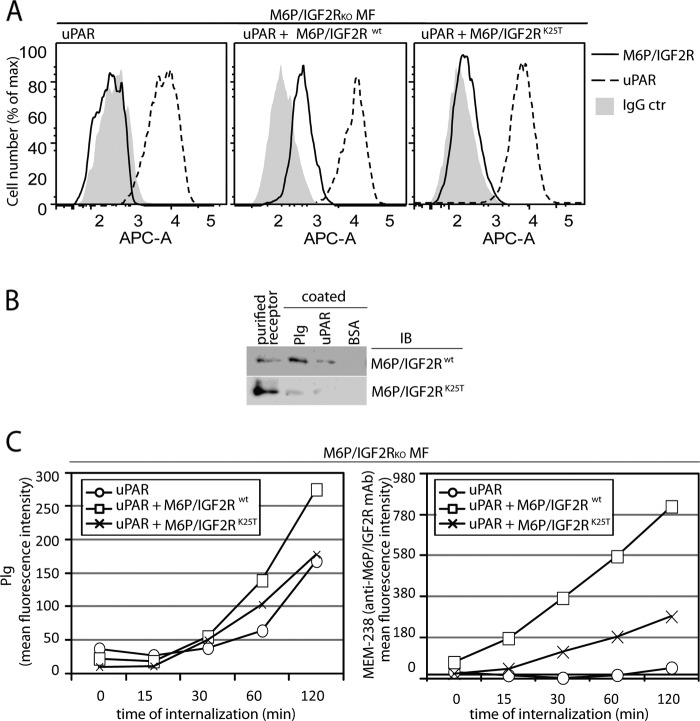
FIGURE 5.
Effect of M6P-IGF2R silencing on Plg internalization in mouse fibroblasts. A, shown is characterization of uPAR/M6P-IGF2R mouse fibroblast (MF) transductants. A retroviral system was used for gene transfer and expression of human M6P-IGF2Rwt and human M6P-IGF2RK25T in murine M6P-IGF2R-knock out mouse fibroblasts (M6P-IGF2RKOMF) expressing human uPAR. Cell surface expression of the receptors was verified by flow cytometry using mAb specific to M6P-IGF2R (mAb MEM-238-APC) and uPAR (H2-AF647). B, for the binding assay, 5 μg/ml purified Glu-Plg or soluble uPAR were coated on wells of a plastic plate in PBS. Then the wells were blocked with BSA and incubated for 4 h on ice with binding buffer supplemented with 5 μg/ml purified M6P-IGF2Rwt or M6P-IGF2RK25T. Bound material was analyzed by SDS-PAGE, and immunoblotting (IB) with mAb specific to M6P-IGF2R (mAb MEM-238) is shown. C, pre-cooled murine M6P-IGF2R-negative mouse fibroblasts expressing human uPAR and M6P-IGF2Rwt or M6P-IGF2RK25T were incubated for 20 min on ice with fluorescently labeled Plg-AF488 (100 nmol/liter) and the anti-M6P-IGF2R MEM-238-APC (5 μg/ml) and then incubated for various time intervals at 37 °C. After acid stripping, the cells were analyzed by flow cytometry for the remaining molecules. Geometric mean fluorescence intensity values are shown as a measure of internalization.