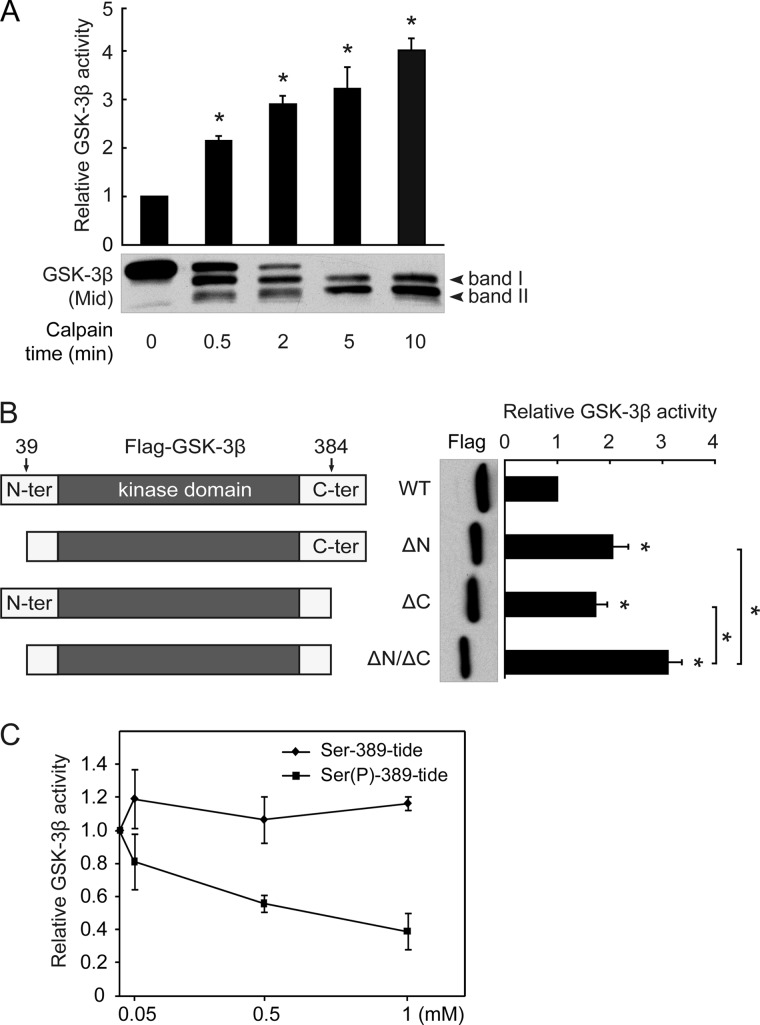
FIGURE 5.
Removal of either N- or C-terminal autoinhibitory domain by calpain activates GSK-3β. A, shown is the effect of calpain-mediated cleavage on the activity of GSK-3β. Recombinant His-tagged GSK-3β was first cleaved by calpain, after which GSK-3β activity was assayed. B, schematic diagrams of the FLAG-GSK-3β WT, ΔN, ΔC, and ΔN/ΔC constructs (left panel) are shown. These constructs were transfected into HEK293 cells, and the resulting cell lysates were immunoprecipitated with the FLAG antibody. The precipitates were subsequently subjected to a GSK-3β kinase assay. C, recombinant GSK-3β was preincubated with the indicated concentrations of Ser-389-tide or Ser(P)-389-tide for 5 min, and a GSK-3β activity assay was performed with 2B-SP as the substrate. All data in this figure represent the mean ± S.E. of at least three independent experiments. *, p < 0.05.