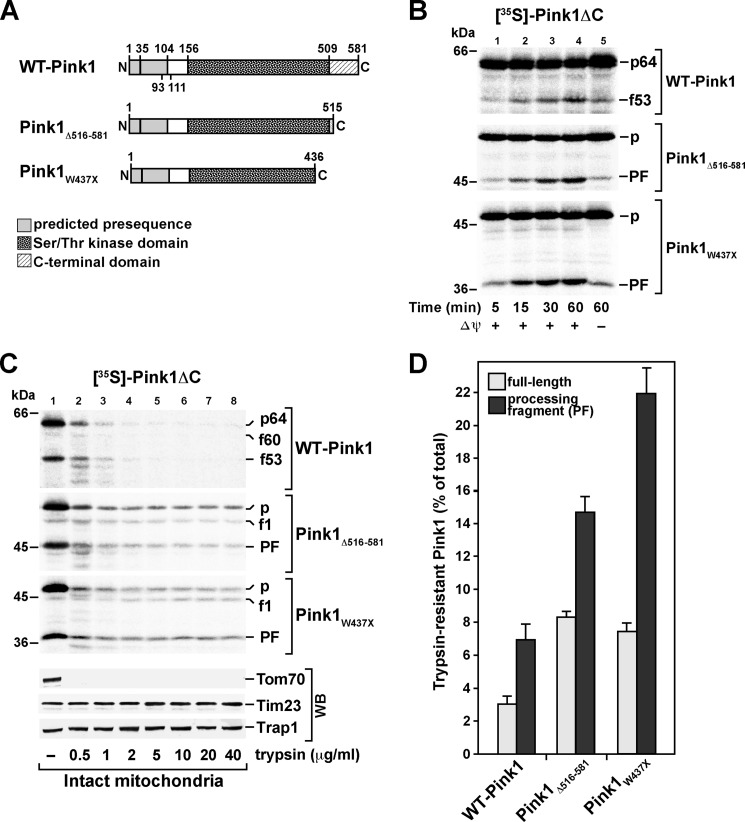
FIGURE 6.
Import, processing, and submitochondrial localization of 35S-labeled C-terminal Pink1 deletion constructs. A, schematic representation of the wild-type Pink1 domain structure, of a C-terminal Pink1 deletion construct lacking the C-terminal 66 residues (Pink1Δ516–581), and of the PD-related Pink1W437X mutant. For wild-type Pink1, previously suggested processing sites consistent with the apparent molecular weight of Pink1f60 and Pink1f53, respectively (positions 35 and 104), and the proposed TMD (residues 93–111) are indicated. B, import of radiolabeled wild-type Pink1 and C-terminal deletion mutants into isolated HeLa mitochondria as described for Fig. 1 with subsequent separation of samples by SDS-PAGE and analysis by digital autoradiography (p, full-length precursor protein; PF, processing fragment corresponding to Pink1f53). C, protease titration of mitochondria following import of wild-type Pink1 (upper panel), Pink1Δ516–581 (middle panel), and Pink1W437X (lower panel). After the import reaction, mitochondria were incubated with trypsin in the indicated concentrations. As a control, immunodecorations against the endogenous proteins Tom70, Tim23, and Trap1 were carried out (p, full-length precursor protein; f1, intermediate processing fragment corresponding to Pink1f60; PF, processing fragment corresponding to Pink1f53). D, quantification of the trypsin resistance of radiolabeled full-length forms and the main cleavage fragments (processing fragment) of WT-Pink1 and the C-terminal deletion constructs after import in intact mitochondria. Autoradiography signals for the indicated Pink1 fragments in the presence of 40 μg/ml trypsin were quantified and depicted as percentage of the imported amounts without protease treatment. Mean values and S.E. were determined for n = 4 independent experiments. WB, Western blot.