FIGURE 6.
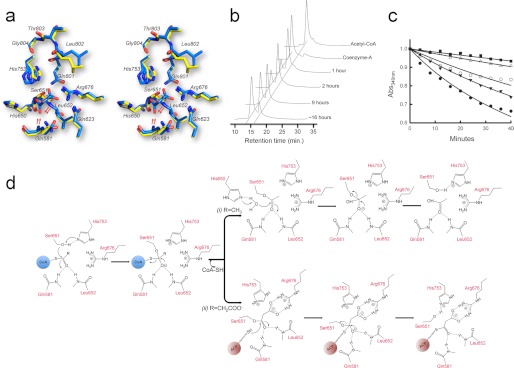
The active site of ATDYN and the catalytic mechanism for acyltransfer. a, stereo view of a superposition of the active site for the free enzyme (yellow sticks) and malonate-bound ATDYN (blue sticks). Residues His753 and Ser651 form the catalytic dyad. Compared with their positions in the unliganded structure, the backbone amides of residues Gln581 and Leu652 are shifted by about 3 Å to shape the oxyanion hole (the movement is indicated by red arrows). Note that the malonate-bound Ser651 rotates downwards by about 30° (also indicated by red arrows). b, HPLC analysis of ATDYN-catalyzed hydrolysis of acetyl-CoA. The HPLC chromatograms show the depletion of the acetyl-CoA substrate along with the increment of the CoA by-product. c, substrate competition inhibition assay. The absorption spectra of NADPH is monitored using UV-visible spectrophotometry at an absorbance of 340 nm. The positive control (black circles), with only 2.5 mm malonyl-CoA as substrate, shows depletion of NADPH, whereas the reaction with the addition of 1.0 mm (black triangles), 2.0 mm (white circles), 2.5 mm (white triangles), and 5.0 mm (black square) acetyl-CoA shows slower depletion of NADPH with increasing concentrations of acetyl-CoA. d, proposed events following post-catalytic mechanism for acetyl- and malonyl-enzyme intermediate. i, acetyl-ATDYN intermediate: His650 deprotonate the adjacent water molecule and subsequently hydrolyzes the covalent bond of acetyl-Ser651. ii, malonate-ATDYN intermediate. A phosphopantetheined-ACP domain extracts malonate by breaking the malonyl-Ser651 covalent bond.