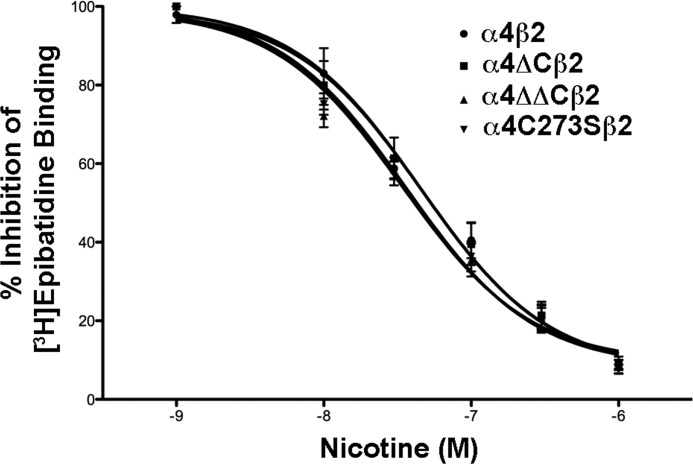
FIGURE 5.
Cysteine mutations in the α4 nAChR subunit do not affect α4β2 nAChR ligand binding affinity. Cultured tsA 201 cells expressing the various α4 constructs plus β2 were fixed with paraformaldehyde, permeabilized, and incubated with 400 pm epibatidine in the presence or absence of 10, 30, 100, 300, or 1000 nm nicotine overnight at 4 °C. After several washes, the amount of bound epibatidine was quantified by scintillation counting. The data shown was normalized to the amount bound in the absence of nicotine and represent the mean ± S.E., n = 6 for α4; n = 5 for α4ΔC; n = 3 for α4ΔΔC and α4C273S. Curves were fitted to a one-site competitive binding using a Kd = 0.015 nm, using the equation Y = 100/(1 + 10((log IC50 − X)·HS), where Y is the percentage of the maximal effect at a given concentration (X), and HS is the Hill Slope (Hill slope = 1).