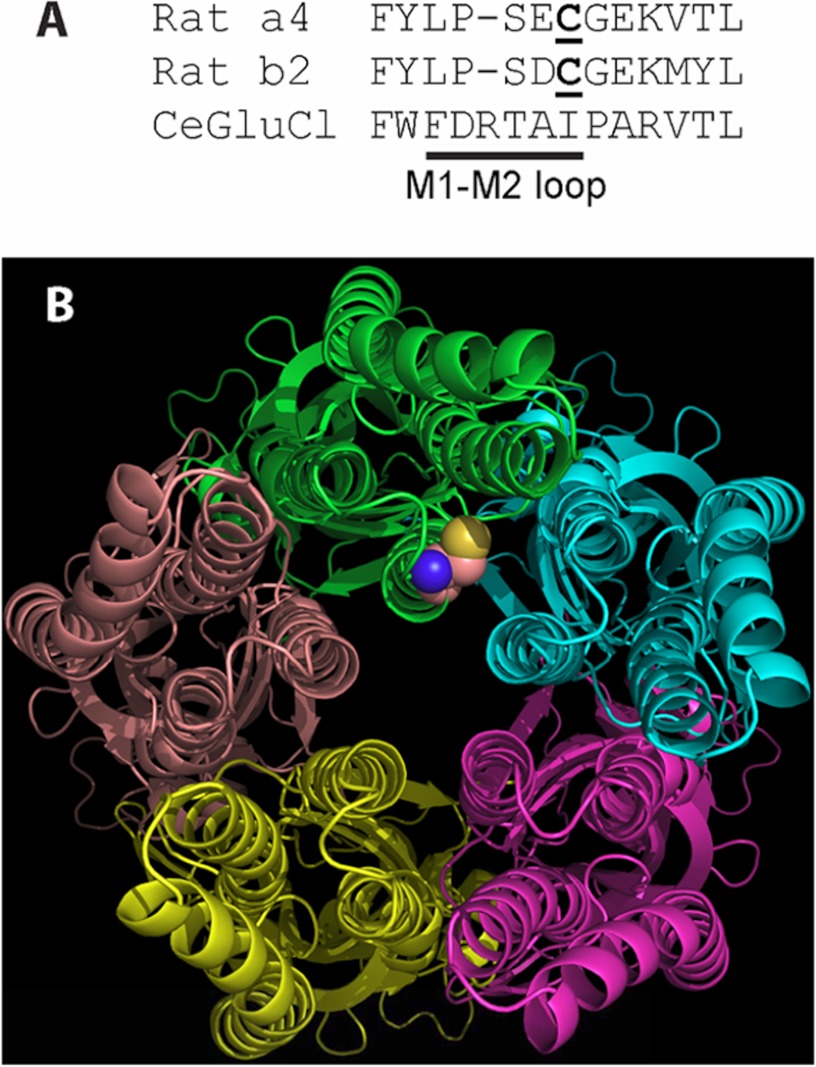
FIGURE 7.
The position of the palmitoylated cysteine likely allows ready access by the hydrophobic palmitoyl moiety to the hydrophilic cytoplasmic space and ion permeation path but does not allow ready access to the lipid bilayer. A, the primary sequence comparison for α4, β2, and a Cys-loop receptor from C. elegans (19) shows the residues of and adjacent to the M1-M2 loop. The palmitoylated cysteine of α4 and homologous cysteine of β2 are underlined. The spatial position of α4Cys273 in B is based on this primary sequence homology and the x-ray crystal structure of the receptor from C. elegans (Protein Data Bank code 3RHW). B, as seen from the cytoplasmic side of the receptor from C. elegans, the likely position of α4Cys273 is adjacent to the transmembrane domains, the cytoplasmic space, and the ion permeation path at the center of the figure. Only the main chains of the subunits are displayed except for the homologous position of α4Cys273, which is displayed as a space-filling model of Cys at the distal end of the M1-M2 loop of the green chain. M1 is at the 9 o'clock position of the green chain, M2 is at 6 o'clock, and M3 is at 3 o'clock.