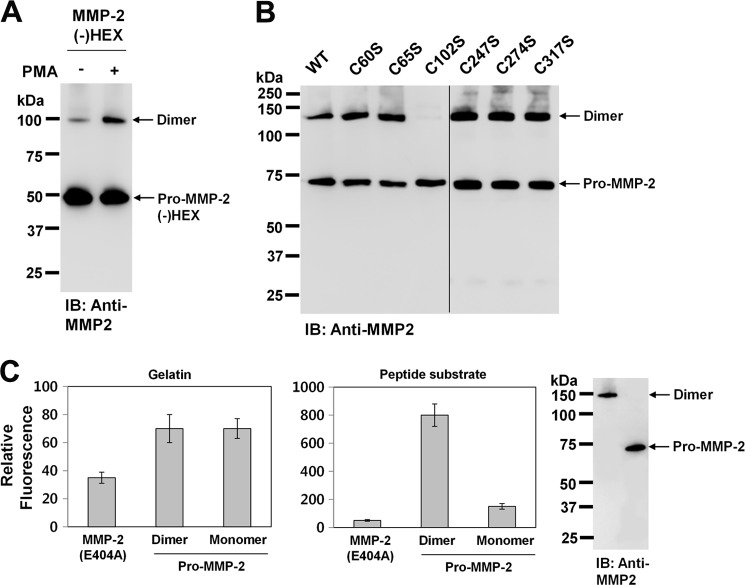
FIGURE 5.
MMP-2 forms a homodimer through an intermolecular disulfide bond. A, Western blot analysis using anti-MMP-2 antibody of the conditioned medium from cells expressing hemopexin-like domain-deleted pro-MMP-2 (MMP-2(−)HEX). Cells were treated with PMA (10 ng/ml) for 16 h. Arrows indicate the monomeric and dimeric forms of hemopexin-like domain-deleted pro-MMP-2 (n = 2). IB, immunoblot. B, Western blotting using anti-MMP-2 antibody of the conditioned media from cells expressing wild-type pro-MMP-2 (WT) and mutants C60S, C65S, C102S, C247S, C274S, and C317S (n = 3). C, digestion assays using fluorescein-conjugated gelatin and MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2. MMP-2 was first purified from the conditioned medium of cells expressing pro-MMP-2 treated with PMA (10 ng/ml) using gelatin-Sepharose and subsequently separated into monomeric and dimeric MMP-2 on Superdex 200 HR. The digested substrates were analyzed as described under “Experimental Procedures.” Data represent the mean ± S.D. of five experiments. The results from Western blot analysis of purified monomeric and dimeric MMP-2 using anti-MMP-2 antibody are shown on the right.