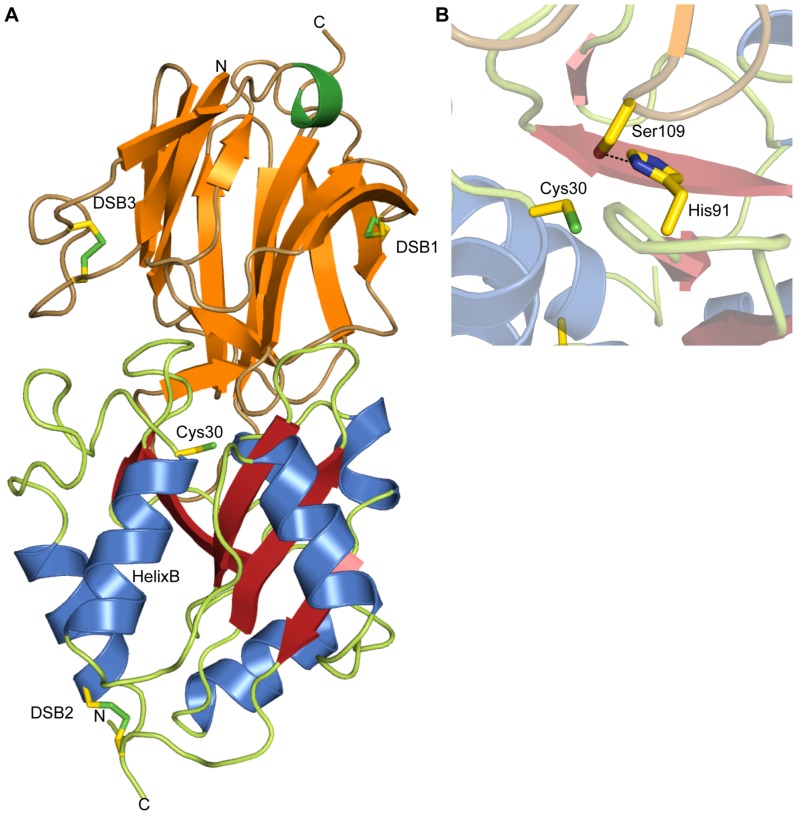
Figure 5. Crystal structure of the Tse1/Tsi1 complex and inhibition of Tse1 by Tsi1.
(A) Crystal structure of the Pseudomonas aeruginosa Tse1/Tsi1 complex using the color scheme of figure 1 for Tse1. Tsi1 is formed by three antiparallel β-sheets (orange) arranged as a partial β-propeller, and a short C-terminal α-helix (green). The N-terminal β-sheet consists of the β-strands β1, β2, β3, β5, and β6. The central β-sheet is made of the β-strands β1, β8, β9, β10, and β11. The C-terminal β-sheet is formed by the β-strands β12, β13, and β14. Residues and disulfide bonds (DSB) in Tse1 (DSB2) as well as in Tsi1 (DSB1 and 3) are depicted as sticks. (B) Close-up of the interaction between Tse1 and Tsi1 at the Tse1 active site. Tsi1 inserts the Ser109 side chain into the Tse1 active site, forming a hydrogen bond to the catalytic His91, keeping it from deprotonating Cys30.