Abstract
Gene-targeted deletion of the voltage-gated potassium channel, Kv1.3 (Kv1.3−/−), increases olfactory sensitivity and discriminatory ability, and causes resistance to diet-induced obesity (DIO) in mice. The objective of the present study was to determine if the enhanced olfactory ability of the Kv1.3−/− mouse contributes to the resistance to DIO. Kv1.3+/+ and Kv1.3−/− mice were subject to bilateral olfactory bulbectomy (OBX) or sham surgery at 9 weeks of age and placed on either a control chow diet (CF) or a 32% moderately high-fat diet (MHF). Caloric and water intake, locomotor activity, and oxygen consumption were monitored after 5 weeks of diet treatment. At the end of 26 weeks of diet treatment, fat pad weight and blood chemistry were evaluated. Kv1.3+/+ mice exhibited a significant increase in weight, adiposity, fasting glucose and fasting leptin in response to the MHF-diet, with or without OBX. When treated with a MHF-diet, Kv1.3−/− mice gained significantly less weight than Kv1.3 +/+ mice and exhibited a significant increase in light phase metabolism. OBX of Kv1.3−/− mice prevented the resistance to DIO and concomitant upregulation of light phase metabolism while decreasing dark phase metabolism and total energy expenditure. These findings suggest that pathways activated in Kv1.3 −/− that increased energy expenditure and led to resistance to DIO are olfactory bulb dependent. Thus, these findings add to a growing body of evidence suggesting that the olfactory system can modulate pathways involved in the regulation of energy balance.
Keywords: Olfactory bulbectomy, diet-induced obesity, super-smeller, potassium channel, oxygen consumption
Introduction
Kv1.3 is a member of the Shaker-like family of potassium-selective, voltage-sensitive ion channels. In the olfactory bulb, Kv1.3 participates in many traditional and non-traditional roles of potassium channels. Kv1.3 carries 60 to 80% of the outward potassium current in mitral cells (1), the primary output neurones of the olfactory bulb, where it depresses the resting membrane potential, decreases firing frequency, increases the latency to first spike, and regulates action potential shape (2–4). Mitral cells from mice with a gene-targeted deletion of Kv1.3 (Kv1.3−/−) are more sensitive to current-evoked stimulation and have modified channel, kinase and adaptor protein expression levels when compared to wildtype (WT) (3–5). The olfactory sensory neurones of these mice are fewer in number, express more olfactory signal transduction machinery per neurone, and project to smaller, supernumerary synaptic units, called glomeruli, within the olfactory bulb (6). Behaviourally, gene-targeted deletion of Kv1.3 results in a “Super-smeller” phenotype characterised by increased sensitivity and discriminatory ability (3). Humans with single nucleotide polymorphisms (SNPs) in the promoter region of the Kv1.3 gene that result in increased expression or enhanced activity of Kv1.3 exhibit olfactory deficits (7).
Kv1.3 also plays an important role in energy balance. Kv1.3−/− mice are thinner than their WT counterparts, yet are normophagic (3,8). Moreover, Kv1.3−/− mice are resistant to weight gain when placed on a moderately high-fat (MHF) diet due to increases in basal metabolic rate (8). When Kv1.3−/−mice are bred to homozygosity to a mouse model of genetic obesity, the melanocortin 4 receptor-null mouse (MC4R−/−), the resulting double homozygous-null mice (MC4R−/−/Kv−/−) gain significantly less weight via an increase in metabolic mass-specific activity-dependent metabolic rate and total energy expenditure (9).
These phenotypic characteristics of the Kv1.3−/− mice suggest that the olfactory system may participate in the regulation of metabolism. Several studies have reported that olfactory stimuli can modulate autonomic outflow and metabolism (10–14). The effects of olfactory stimulation have a circadian component, whereby olfactory stimulation is effective only during the light phase, resulting in modulation of basal metabolic rate only, as measured by changes in body temperature and intrascapular brown adipose tissue temperature (15). Chronic olfactory stimulation, 15 minutes per day, 3 days a week for 6 weeks, results in a significant decrease in caloric intake and body weight with grapefruit oil (11) and conversely, an increase in caloric intake and body weight with lavender oil stimulation (12). Bilateral lesions of the main olfactory epithelium (which results in complete anosmia) or suprachiasmatic nucleus (SCN) (14–16) disrupt the autonomic responses to olfactory stimulation.
These observations lead to the hypothesis that the diet-induced upregulation of basal metabolism of Kv1.3−/− mice is due to the increased sensitivity of the olfactory system of these “Super-smeller” mice. To evaluate the importance of increasing the sensitivity of the olfactory bulb by decreasing Kv1.3 activity, bilateral olfactory bulbectomy was used to make the “Super-smeller” Kv1.3−/− mice chronically anosmic. As a result, we have found that the resistance to weight gain and the upregulation of basal metabolism in response to MHF-diet normally observed in Kv1.3−/− mice is olfactory bulb dependent.
Materials and Methods
Animal Care and Mouse Lines
All mice were housed at the Florida State University vivarium in accordance with the institutional requirements for animal care. Kv1.3-null mice (Kv1.3−/−) were a generous gift from Drs. Leonard Kaczmarek and Richard Flavell (Yale University, New Haven, CT) and were generated by excision of the Kv1.3 promoter region and one third of the 5’ coding region in a C57BL6/J background (17). Male wild-type (C57BL6/J; Kv1.3+/+) and Kv1.3−/− mice were maintained on a standard 12/12h light/dark cycle with ad libitum access to 5001 Purina Rodent chow (CF; 13.5% kcal fat) and water until eleven weeks of age, at which point, half were fed a moderately high fat (MHF), 32% kcal fat, condensed milk diet from Research Diets (D12266B; New Brunswick, NJ). Body weight and caloric intake were monitored biweekly.
Bilateral Olfactory Bulbectomy
Nine-week-old male mice were anesthetized in accordance with the National Institutes of Health (NIH) and the Florida State University Animal Care and Use Committee procedures by an intraperitoneal injection of 100 mg/kg ketamine and 8 mg/kg xylazine. A 1.5 cm midline incision was made between the eyes and then the scalp was deflected to expose the cranial covering of the olfactory bulbs. Two 1.5 mm holes (one over each olfactory bulb) were drilled through the skull, using a Dremel tool with a sterile #106 bit to expose the underlying olfactory bulb tissue. Sham olfactory bulbectomy surgery ended at this point after closing the scalp incision with Vetbond Tissue Adhesive (3M Pet Care Products). For bilateral olfactory bulbectomy (OBX) treatment, a sterile p10 Pipetman tip (Fisher Scientific) connected to a suction hose and collection flask was used to aspirate the olfactory bulb tissue from the cavity. The cavity was then filled with sterile Gel Foam (UpJohn, Kalamazoo, MI) as previously described (18) and the overlying scalp incision was closed using Vetbond Tissue Adhesive. Surgically-treated animals were allowed to recover for two weeks prior to placement on a dietary regime at eleven weeks of age. Animals were maintained on a control or MHF-diet for 26 weeks following surgery recovery.
Behavioural and Histological Confirmation of Olfactory Bulbectomy
To evaluate the success of the OBX surgery, animals were subjected to a general anosmia test as previously described (3) by measuring the time taken to find a hidden chocolate-scented candy (Whopper) and comparing it to the time taken to find a hidden, unscented marble. Each item was tested three times per subject. The resultant times were averaged within an animal per item and compared statistically with a Students t-test. If the average time taken to find the scented item was significantly lower than that of the unscented marble, indicating the animal could smell, the subject was suspected to have an incomplete OBX and after histological confirmation, was discarded from the study.
The behavioural results were confirmed by post mortem histological examination of the remaining olfactory bulb tissue or cavity. Post-fixed heads were decalcified in 0.3 M EDTA for 3 to 5 days at 4°C. The decalcified heads were cryoprotected overnight in 10% sucrose in PBS followed by overnight incubation in 30% sucrose in PBS. Cryosections were prepared using a Cryostat Microtome model HM 500 OMV (Microm GmbH, Waldorf, Germany). Sixteen micron thick horizontal sections were affixed to gelatin-coated slides, and stored at 20°C until use. The tissue sections were incubated in 0.1% Neutral Red for 15 min followed by four successive washes in distilled water, two 30 s washes in 100% ethanol and a 10 minute xylene wash. The extent of olfactory bulb removal was visualized with a Nikon MicroPhot-FXA microscope (Nikon Inc., Mellville, NY) and documented with a Fast 1394 Qucam digital camera (Qimaging, Surry, BC, Canada). If an OBX animal was not found to be anosmic or had more than 25% olfactory bulb tissue remaining, that animal was excluded from analysis. Using these two metrics, a total of 4 of 44 mice were excluded from our study.
Indirect Calorimetry and Behavioural Monitoring
Oxygen consumption (VO2; ml/min) and CO2 production (VCO2; ml/min) of individually housed mice were continuously monitored for eight days at 23°C in custom-fabricated metabolic isolation chambers as previously described, five to seven weeks after the beginning of diet treatment (9,19,20). In brief, mice were placed in shoebox cages (26 x 47 x 13.5 cm) fitted with near-air tight lids that received fresh air at a rate of 0.5 L/min. Dry, mixed-cage air was sampled with a 250 ml/min flow rate and sent to O2 and CO2 gas analyzers every four minutes (min) for 30 seconds (s) followed by VO2 and VCO2 determination by open-circuit respirometry according to Bartholomew and colleagues (21), with modifications to isolate successive samples (19). All reported mean values were calculated for 12 hours of dark and 11 hours of light so that daily body weight, caloric intake, and water consumption could be measured during the last hour of the light cycle. Due to technical restraints in the measurement and interpretation of open-circuit indirect calorimetry of small animals, we have reported both uncorrected (Supplemental Material S3) and weight-corrected oxygen consumption (Figure 4) in the light and dark phase and compared linear regressions of uncorrected oxygen consumption to weight with an Analysis of Covariance (ANCOVA) as previously suggested (22)(Supplemental Material S3). Each cage was positioned on a custom-designed platform resting on a centred fulcrum with stiff strain-gauge load-beam transducers positioned under two adjacent corners to measure animal position and locomotor activity as previously described (9,20). Locomotor activity was reported in metres, binned every 30 s, and measured at a 1 mm resolution. Daily caloric intake was calculated by measurement of consumed powdered Purina 5001 rodent chow (3.3 kcal/g) or MHF rodent chow (4.41 kcal/g). Dark and light phase energy expenditure were calculated using the Weir equation (23): . Total energy expenditure (TEE) was calculated as the sum of both the dark- and light-phase EE. As with oxygen consumption, we have reported uncorrected (Supplemental Material S4) and weight-corrected TEE (Figure 6) and compared linear regressions of uncorrected TEE to weight with an ANCOVA (Supplemental Material S4).
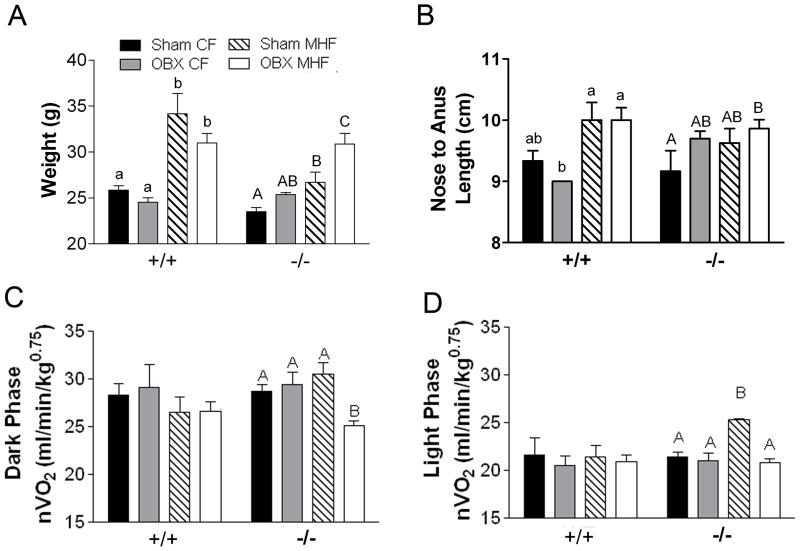
Figure 4.
OBX prevents the increased basal-metabolic response of Kv1.3−/− mice to the MHF-diet challenge. A) Bar graph of body weight at the time of metabolic monitoring, approximately 5–7 weeks after initiation of diet treatment. B) Average nose to anus length indicating overall growth changes. C) dark phase or activity-dependent metabolism and D) light phase or basal metabolism normalized to metabolic mass (nVO2). Lettering style, statistical analysis, and abbreviations as in Figure 2. Values represent mean ± SEM of 2 day averages within an animal, n=5.
Figure 6.
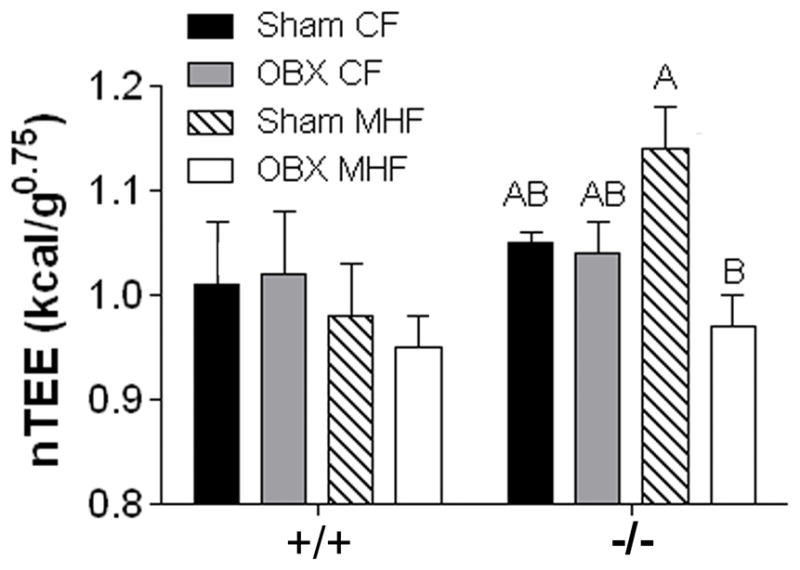
Olfactory bulbectomy reduces metabolic mass-specific energy expenditure in MHF-treated Kv1.3−/− mice. Bar graph of metabolic mass-specific total energy expenditure (nTEE). Lettering, statistical analysis, and abbreviations as in Figure 2. All values represent mean ± SEM of 2 day averages within animal.
Fat Pad and Serum Collection
After 26 weeks of control or MHF diet treatment, mice were fasted overnight, measured from nose to anus, and then weighed. Mice were then anesthetized with isofluorane inhalation followed by decapitation in accordance with NIH and Florida State University Animal Care and Use Committee approved methods. Fasting glucose, insulin, and leptin levels in trunk blood were determined as previously described (9). Briefly, an Ascensia Contour Blood Glucose Monitoring System (Bayer Healthcare, Mishawaka, IN) was used immediately following decapitation to measure blood glucose. Serum was collected as previously described (9) and stored at −20°C for later examination with a Mouse Leptin ELISA Kit (Linco Research, St. Charles, MO) and an Ultrasensitive Mouse Insulin ELISA Enzyme Immunoassy (Mercodia AB, Uppsala, Sweden) to determine fasting serum levels of leptin and insulin, as per manufacturer’s protocols. The skull was stripped of all external muscle and tissue and fixed in 4% formaldehyde in phosphate buffered saline at 4°C overnight for histological processing as described above. All visceral fat pads, including epididymal, retroperitoneal, and mesenteric white adipose tissue (WAT) were removed from the abdominal cavity, separated, and weighed. Subcutaneous WAT was sub-sampled by weighing the fat pad on the right side of each animal, from the median line of the abdomen to the spine and the right hip to the first rib. Brown adipose tissue was removed from between the scapulae and weighed.
Data Analysis and Statistics
Metabolism, energy expenditure, locomotor activity, caloric intake, and water consumption values were two-day averages after five to six days of acclimation of the animal to the metabolic chambers. Statistical significance was determined using either a Student’s t-test or a two-way analysis of variance (ANOVA) across diet and surgery within a genotype using a Student Newman Keuls (snk) or Bonferroni post-hoc test, as indicated in text, at the 95% confidence interval. For both oxygen consumption and TEE, we have also compared linear regressions of uncorrected TEE and oxygen consumption to weight with an ANCOVA. These ANCOVA comparisons provide appropriate statistical comparisons across animals of significantly different weights in the absence of fat free mass measurements. An effect was considered significantly different if p<0.05 for a difference in elevation.
Results
Kv1.3 Gene-targeted Deletion Prevents Diet-induced Obesity
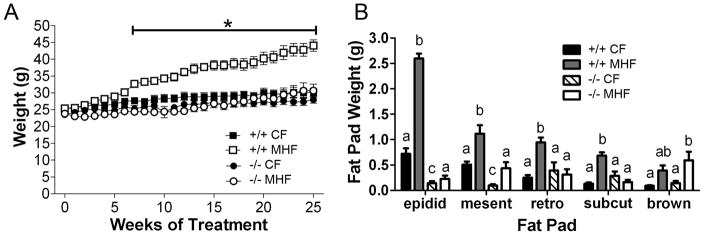
To confirm the extent to which Kv1.3−/− mice are resistant to diet-induced obesity, mice were fed either a control diet (13.5% Kcal fat; CF) or a moderately high fat diet (32% Kcal fat; MHF) starting at 11 weeks of age through 37 weeks of age. Kv1.3+/+ mice on the MHF-diet gained significant weight, beginning at week eight, continuing throughout treatment whereas, Kv1.3−/− mice did not (Figure 1A). Kv1.3+/+ mice fed the MHF-diet had a significant increase in all fat pads examined except brown fat (Figure 1B). Kv1.3−/− mice exhibited only an increase in mesenteric and brown fat when challenged with the MHF-diet (Figure 1B ). Fasting glucose levels were not changed in Kv1.3+/+ mice with MHF-diet treatment while glucose levels dropped in Kv1.3−/− mice in response to the MHF-diet (Supplemental Material S1, left panel). Fasting insulin levels were significantly higher in Kv1.3+/+ mice in response to a MHF-diet while there was no change in Kv1.3−/− mice (Supplemental S1, middle panel). MHF-diet treatment caused a significant elevation of leptin in Kv1.3+/+ but not Kv1.3−/− mice (Supplemental S1, right panel).
Figure 1.
Kv1.3-null mice are resistant to diet-induced obesity. (A) Line graph plot of weekly body weight for WT (+/+) and Kv1.3 knockout (−/−) mice throughout 26 weeks of either a moderately high fat (MHF; 32% Kcal fat diet) or control-fat (CF; 13.5% Kcal fat diet) dietary treatment. Treatment week 0 represents the weight of the animals the week before treatment began (11 weeks of age). Line and asterisk indicate significant differences as determined by a two-way analysis of variance (diet vs. genotype) at the 95% confidence interval, followed by a Bonferroni post hoc test. Values represent mean ± SEM, n=5 for each treatment/genotype combination. (B) Bar graph of various fat pads (epidid = epididymal; mesent = mesenteric; retro = retroperitoneal; subcut = subsampled subcutaneous; brown = intrascapular brown fat) as indicated, for +/+ and −/− after 26 weeks of diet treatment. The lowercase letters indicate significant differences within each fat pad as determined by a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. NOTE: If the letters are different, the values are significantly different (example: a is different from b and c, but ab is not different from a or b).
Kv1.3−/− Mice are no Longer Resistant to Diet-induced Obesity Following Olfactory Bulbectomy
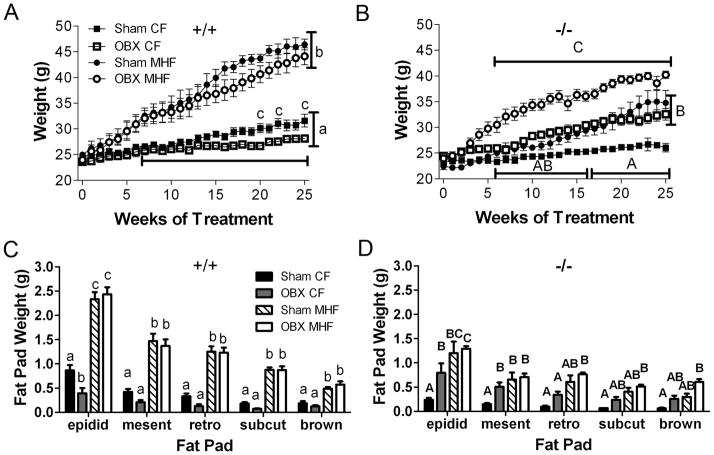
To determine if the resistance to diet-induced obesity observed in Kv1.3−/− mice is due to their increased olfactory sensitivity or something intrinsic to the olfactory bulb and its efferent projections, we performed sham or bilateral olfactory bulbectomy (OBX) surgeries at 9 weeks of age. After a two week recovery period, mice were fed either a CF- or MHF-diet for 26 weeks. OBX alone decreased body weight and epididymal adiposity in Kv1.3+/+ mice by 15 weeks of CF-diet treatment, however, OBX did not prevent or significantly reduce weight gain or adiposity during the MHF-diet treatment (Figure 2A and C). In Kv1.3−/− mice, OBX alone had the opposite effect in that body weight significantly increased by 10 weeks for CF-diet animals and epididymal and mesenteric adiposity was significantly increased by 26 weeks (Figure 2B and D). Furthermore, removal of the olfactory bulbs resulted in weight gain and increased adiposity of Kv1.3−/− mice challenged with a MHF-diet (OBX MHF), completely reversing the effect of the Kv1.3 gene-targeted deletion on resistance to diet-induced weight gain (Figure 2B and D). Sham-operated Kv1.3−/− mice maintained on a MHF-diet were not significantly different in weight and adiposity from OBX-operated mice maintained on a CF diet, yet they were significantly higher than unoperated Kv1.3−/− mice (Figure 1) while being significantly lower than OBX-operated Kv1.3−/− mice on the MHF diet (Figure 2). This intermediate phenotype in the Kv1.3−/− mice could be due to differences in inflammatory responses to surgery or potential disruption of the blood brain barrier.
Figure 2.
Kv1.3−/− mice are no longer resistant to diet-induced obesity following olfactory bulbectomy. (A and B) Line graph of weekly body weight and (C and D) bar graph of various fat pad weights. A and C = +/+; B and D = −/− mice after olfactory bulbectomy (OBX) or sham surgery (Sham). Diet treatments and abbreviations as in Figure 1. Values represent mean ± SEM, (A-D n=5–8; E n=4–5). In A-D: lowercase letters indicate significant differences within +/+ mice and uppercase letters indicate significant differences within −/− mice as determined by a two-way analysis of variance (2-way ANOVA; within a genotype for treatment vs. time) at the 95% confidence interval, followed by a Bonferroni post hoc test. In C-D: lowercase letters indicate significant differences within +/+ mice and uppercase letters indicate significant differences within −/− mice as determined by a one-way analysis of variance (ANOVA; within a fat pad or a genotype) at the 95% confidence interval, followed by a Bonferroni post hoc test.
At the end of the 26 weeks of diet treatment, there were no significant differences detected in Kv1.3−/− mice for fasting glucose or insulin (Supplemental Material S2) compared with what was observed for unoperated animals. In Kv1.3+/+ mice, glucose and leptin levels were elevated with MHF-diet with either surgical treatment, but insulin was only significantly elevated with a combination of both MHF and OBX treatment (Supplemental Material S2). Bulbectomy alone and in combination with MHF-challenge now caused a significant elevation of leptin in Kv1.3−/− mice (Supplemental Material S2) compared with the MHF-challenged, unoperated mice.
Olfactory Bulbectomy Transiently Increases Caloric Intake
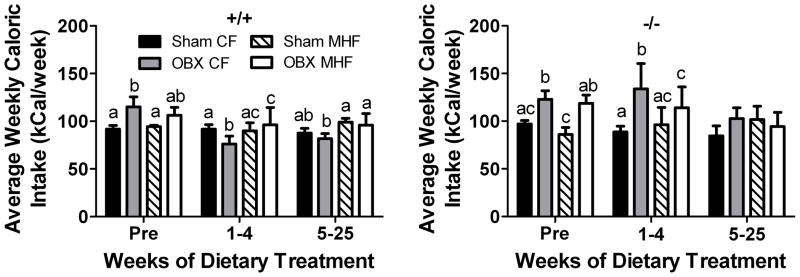
Caloric intake was monitored throughout the 26 weeks of the feeding treatment as well as one week prior to the beginning of diet treatment and one week post-surgical treatment (Figure 3). Both Kv1.3+/+ and Kv1.3−/− OBX mice had higher caloric intake the week before diet treatment began that persisted in CF but not MHF fed Kv1.3+/+ mice (Figure 3, left panel) and both CF and MHF fed Kv1.3−/−mice (Figure 3, right panel) for the first four weeks of diet treatment. After the first five weeks of diet treatment all mice had similar weekly caloric intake regardless of caloric source.
Figure 3.
Olfactory bulbectomy transiently increases caloric intake. Bar graph of average weekly caloric intake of (A) +/+ and (B) −/− mice following olfactory bulbectomy or sham surgery (sham). Diet treatments and abbreviations as in Figure 1. Treatment week Pre represents the caloric intake of the animals the week before the feeding treatment began and therefore all animals were receiving CF-diet. Values represent mean ± SEM, n=5–8. Letters indicate significant differences as in Figure 2, as determined by a two-way analysis of variance (2-way ANOVA; treatment vs. diet for each time interval) at the 95% confidence interval, followed by a Bonferroni post hoc test.
Olfactory Bulbectomy Prevents the MHF-diet Induced Increase in Basal Metabolic Rate in Kv1.3−/− Mice
To examine the underlying metabolic and/or behavioural changes that contribute to the adiposity phenotypes, OBX, mice were placed in custom-built metabolic chambers after five to seven weeks of diet treatment (seven to nine weeks post surgery). Dark and light phase oxygen consumption (VO2) or metabolism, and locomotor activity were monitored for eight days (four days of acclimation and four days of data collection). At the time of metabolic monitoring, the weight of animals across treatments was significantly different (Figure 4A) and by the end of the 26 weeks, the overall nose to anus lengths (Figure 4B) became significantly different. Together with the slight mismatch in adiposity and final body weight in the Kv1.3 −/− mice (Figure 2), these results suggest an overall change in growth and lean mass, necessitating some sort of normalization. Due to the established effects of animal size on metabolism and energy expenditure (EE) and the caveats intrinsic to various approaches to normalize metabolism for body mass (22), we choose to report both metabolic mass-specific VO2 (nVO2; ml/min/kg of body weight 0.75; Figure 4C and D) (24), and non-normalized VO2 (VO2; ml/min; Supplemental Material S3A and B). Because we were not able to determine lean mass at the time of indirect calorimetry, non-normalized VO2 (Supplemental Material 3C-F) and TEE (Supplemental Material S4B and C) for sham and OBX MHF-diet treated animals were plotted against weight and subjected to a linear regression. The linear regression lines were then compared with an analysis of covariance to detect changes in elevation as suggested by Arch and colleagues (22). These analyses of covariance measurements, although imperfect when used with low sample number and small variability, corroborate the nVO2 results.
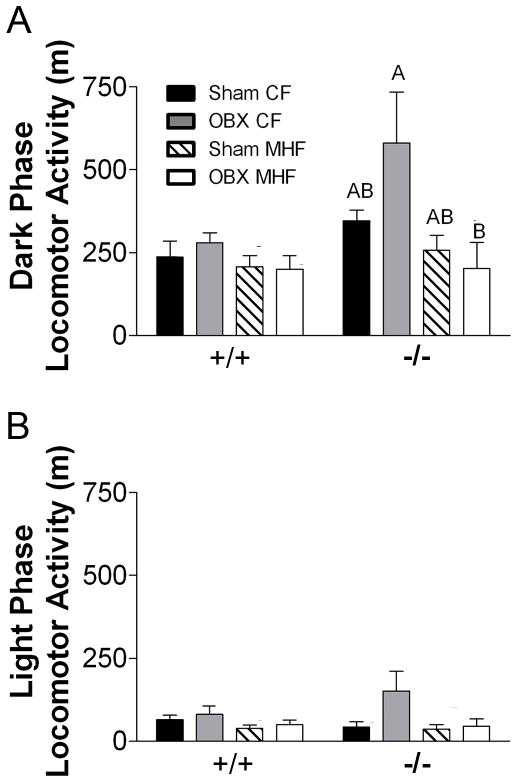
OBX alone does not result in significant changes in dark phase (basal plus activity-dependent) or light phase (basal) nVO2 (Figure 4C and D), VO2 (Supplemental Material S3A and B) or locomotor activity (Figure 5A and B) for either genotype. MHF-diet treatment selectively and significantly increased basal nVO2 in Kv1.3−/− mice but not Kv1.3+/+ mice (Figure 4D) without changing locomotor activity (Figure 5B). OBX prevented the MHF diet-induced increase in basal nVO2 (Figure 4D), as well as diet-induced decrease in the activity-dependent nVO2 (Figure 4C)in Kv1.3−/− mice but not Kv1.3+/+ mice. These results were confirmed by ANCOVA of the linear regression relationships of MHF-diet treated sham and OBX mice for both Kv1.3+/+ (Supplemental Material S3C and D) and Kv1.3−/− mice (Supplemental Material S3E and F). These data show that there are no significant differences for the relationship of weight and VO2 between sham and OBX treatment in MHF fed Kv1.3+/+ mice. However, OBX significantly reduces both the dark and light phase VO2 in Kv1.3−/− mice treated with a MHF-diet without significant changes in locomotor activity (Figure 5A and B).
Figure 5.
Locomotor activity is variable in the dark phase for Kv1.3−/− mice. Bar graphs of (A) dark and (B) light phase locomotor activity of +/+ and −/− mice. All values represent mean ± SEM of 2 day averages within an animal. Abbreviations, lettering, and statistical analysis as in Figure 2.
The collective metabolic changes measured for the Kv1.3−/− mice are attributed to significant changes in metabolic mass specific total energy expenditure (nTEE; kcal/ kg of body weight 0.75; Figure 6). MHF feeding of sham operated Kv1.3−/− mice resulted in a trend (p<0.1) for increased nTEE and a significant increase in TEE when compared to the CF-diet condition (Figure 6, Supplemental Material S4A). OBX treatment significantly decreased nTEE and TEE in Kv1.3−/− mice challenged with a MHF- diet (Figure 6, Supplemental Material S4A and C).
Discussion
It has previously been shown that gene-targeted deletion of Kv1.3 reduces weight gain in a genetic model of obesity by selectively increasing activity-dependent metabolism (9), prevents diet-induced obesity by selectively increasing basal metabolic rate (8) and increases olfactory sensitivity (3). Here, we confirm that Kv1.3−/− mice are resistant to diet-induced obesity as a result of a selective increase in basal metabolic rate. However, bilateral olfactory bulbectomy (OBX), a procedure which results in chronic anosmia or large olfactory deficits, completely blocks the basal metabolic rate upregulation that is normally observed in Kv1.3−/− mice in response to a MHF-diet challenge. By preventing the upregulation in basal metabolic rate and concomitant TEE, OBX Kv1.3−/− mice now exhibit diet-induced obesity.
OBX of rats and mice results in general odorant and semio-chemical anosmia, due to the obligatory removal of both the accessory and the main olfactory bulbs. It is also a model of depression resulting in associative-learning and memory deficits, stress-induced hyperactivity, decreased mobility in the forced swim test, reduced food-motivated behaviours, reduced sexual activity, and differential changes in aggressive behaviours (as reviewed in (25). OBX can also result in energy-balance related changes such as weight loss (26,27), short-term increased caloric intake (27,28), locomotor activity (26), heart rate, and body temperature (29,30). There is also evidence in several rodent species and at least one primate species for the disruption of olfactory bulb-mediated circadian rhythms revealed by OBX surgery (28,30–34).
During the experiments presented here, no gross differences in overall behaviour were observed with the exception of a more docile nature in OBX, MHF-diet treated mice of both genotypes. However, no systematic evaluation of depression-like behaviours were performed. Therefore, it is unknown if OBX induces depression-like symptoms in Kv1.3−/− mice or if those symptoms would manifest differently in these mice. We did observed a decrease in body weight in Kv1.3+/+ mice fed a control fat diet (CF) as a result of OBX treatment similar to that found previously (26). A stress-induced increase in locomotor activity attributed to OBX as previously reported (26,35,36) was not observed in this study. This could have been because the mice were allowed to acclimate to the metabolic chambers for at least four days before data were used for baseline analysis, although OBX-treated Kv1.3−/− mice did tend to be hyperactive in the absence of a MHF-diet. Caloric intake was transiently increased within the first four to seven weeks post OBX in both genotypes regardless of caloric source. It is also interesting that CF-treated Kv1.3−/− mice gained weight as a result of OBX treatment while Kv1.3+/+ mice lost weight under the same conditions.
The olfactory system, including the olfactory bulb, is now being considered not simply as a detector of external chemical cues but of internal metabolic chemical cues such as insulin, glucose, and leptin (4, 37–39). Therefore, regardless of any depressive nature of OBX surgery in rodents, the changes observed with OBX are attributable to removal of intrinsic olfactory bulb mechanisms or perturbation of the bulb’s input to downstream targets. The olfactory bulb projects to many brain regions, either directly or indirectly, that are responsible for regulation of sympathetic and parasympathetic tone and therefore has the opportunity to regulate energy balance. The OB projects directly to the piriform cortex, amygdala, entorhinal cortex, anterior olfactory nucleus, and the taenia tecta (40). The piriform cortex, amygdala, entorhinal cortex, and anterior olfactory nucleus send projections to the dorsal endopiriform nucleus which in turn projects to the suprachiasmatic nucleus (SCN) of the hypothalamus (40). The taenia tecta projects directly to the SCN (40). The SCN is the internal clock that dictates many physiological, behavioural and endocrine rhythms of the body (41,42). Heart rate, blood pressure, metabolism, leptin, insulin and glucose regulation all have a circadian component that are controlled by the SCN via multi synaptic connections from the SCN to the paraventricular nucleus of the hypothalamus (PVN), and then through either the intermediolateral column of the spinal cord to regulate sympathetic tone, or the dorsal motor nucleus of the vagus to regulate parasympathetic activity as reviewed by (43).
In a series of papers that span from 2003 to 2008, Nagai and colleagues, found that odor stimulation can differentially modulate sympathetic and parasympathetic activity to modulate rodent heart rate, blood pressure, lypolysis, caloric intake, body weight, plasma glycerol, and body temperature in an odor-specific manner (11–13,15,16,44) determined by ZnSO4 lesioning and xylocane inactivation of the olfactory epithelium. Olfactory-dependent modulation of these parametres is only effective during the light phase of the circadian cycle and is completely abolished by SCN lesion (15). “Super–smeller” Kv1.3−/− mice have an increase in basal metabolic rate in response to the MHF-diet that is prevented in the anosmic, OBX condition. OBX-treated Kv1.3+/+ mice exhibit no change in either basal or activity-dependent metabolic rate. The olfactory bulb dependent upregulation of basal metabolism of the Kv1.3−/−mice in response to the MHF is similar to those of Nagai and colleagues. Specifically, acute stimulation with grapefruit oil results in increased basal metabolism, not activity-dependent or dark phase metabolism, as measured by core body and intrascapular brown fat temperature (15) and decreased body weight with chronic olfactory stimulation. While ZnSO4 lesioning and xylocane inactivation of the olfactory epithelium would have been good additions to the present study in theory, these manipulations are temporary and not conducive to the chronic design of this study. More permanent olfactory epithelium ablation experiments are necessary to definitively differentiate between olfactory and olfactory bulb specific mechanisms and are planned for future studies.
While our surgical and genetic manipulations represent artificial situations, there are many natural modulators of Kv1.3 activity in the olfactory bulb that may also affect the sensitivity of the olfactory system and thereby modulate sympathetic and parasympathetic output via the SCN, perhaps on a smaller scale. A naturally occurring polymorphism in the promoter region of the Kv1.3 gene in humans, associated with a gain of function, has been correlated with olfactory deficits, insulin insensitivity, and impaired glucose tolerance in humans (7,45). Brain-derived neurotrophic factor (BDNF) and insulin, via activation of their respective tyrosine kinase receptors, suppress Kv1.3 current by tyrosine specific phosphorylation in the olfactory bulb (1,46–51). The cellular tyrosine kinase, Src, as well as the adapter proteins Grb10, PSD-95, and Shc, also modulate Kv1.3 current and are highly expressed in the olfactory bulb (51–53). In making these mice “Super-smellers” through dampening or eliminating Kv1.3 conductance, it is possible that we have increased the innate ability of the olfactory system to modulate sympathetic and parasympathetic output via the SCN. Future studies, in which metabolic rate is monitored in Kv1.3+/+ vs. Kv1.3−/− mice in response to various olfactory stimuli and in the total absence of olfactory input (such as naris occlusion) are needed to understand the fine-tuned role of the olfactory system in energy balance.
Supplementary Material
S1. Blood chemistry of intact Kv1.3 +/+ and −/− mice. Bar graph of fasting blood glucose (left), serum insulin (middle), and leptin (right) concentrations after 26 weeks of diet treatment. The lowercase letters indicate significant statistical differences within each fat pad as determined by a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test.
S2. Blood chemistry of sham and OBX surgically treated Kv1.3 +/+ and −/− mice. Bar graph of fasting blood glucose (left), serum insulin (middle), and leptin concentrations (right) after 26 weeks of diet treatment in sham- and OBX-treated mice. Lowercase letters indicate significant differences within +/+ mice and uppercase letters indicate significant differences within −/− mice as determined by a one-way analysis of variance (ANOVA; within a genotype) at the 95% confidence interval, followed by a Bonferroni post hoc test.
S3. Non-normalized metabolic data and analysis of covariance (ANCOVA). Non-normalized A) dark phase or activity-dependent metabolism and B) light phase or basal metabolism. Values represent mean ± SEM of 2 day averages within an animal, n=5. Scatter plot of metabolic rate versus weight are plotted for both genotypes maintained on the MHF-diet (C,E) = dark phase; (D,F) = light phase. Data were fit by linear regression and the elevation differences were compared by ANCOVA at the 95% confidence interval. Solid line and closed circles = sham MHF; Dashed line and open circles = OBX MHF. If a significant difference was detected in elevation, the p value (p) was reported. No significance is indicated by N.S., n=5. A-B) Lettering style, statistical analysis, and abbreviations as in Figure 2.
S4. Non-normalized total energy expenditure (TEE). A) Bar graphs of non-normalized TEE. Lettering, statistical analysis, and abbreviations as in Figure 2. All values represent mean ± SEM of 2 day averages within animal. Scatter plot of TEE versus weight of (B) +/+ and (C) −/− treated with a MHF-diet. Data were fit by linear regression and the elevation differences were compared by analysis of covariance (ANCOVA) at the 95% confidence interval. Solid line and closed circles = sham MHF; Dashed line and open circles = OBX MHF. If a significant difference was detected in elevation, the p value (p) was reported. No significance is indicated by N.S., n=5.
Acknowledgments
The authors would like to thank Michael Henderson, Jeffery Godby, Chris Kovach, and Patrick Jean-Baptiste for technical assistance. A special thanks to Dr. Jessica Brann for instruction in olfactory bulbectomy surgery and Ross Henderson for engineering support. This work was supported by grants from the US National Institutes of Health (NIH) R01 DC003387, F31 DC010097, and T32 DC00044 from the National Institute of Deafness and Communication Disorders (NIDCD), and the Tallahassee Memorial Hospital/Robinson Foundation. The authors have no conflicts of interest to declare.
References
- 1.Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci. 1998;18:6126–6137. doi: 10.1523/JNEUROSCI.18-16-06126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balu R, Larimer P, Strowbridge BW. Phasic Stimuli Evoke Precisely Timed Spikes in Intermittently Discharging Mitral Cells. J Neurophysiol. 2004;92:743–753. doi: 10.1152/jn.00016.2004. [DOI] [PubMed] [Google Scholar]
- 3.Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1. 3 channel gene-targeted deletion produces "Super-Smeller Mice" with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41:389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadool DA, Tucker K, Pedarzani P. Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1. 3 ion channel. PLoS One. 2011;6:e24921. doi: 10.1371/journal.pone.0024921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu S, Das P, Fadool DA, Kaczmarek LK. The Slack sodium-activated potassium channel provides a major outward current in olfactory neurons of Kv1. 3−/− super-smeller mice. J Neurophysiol. 2010;103:3311–3319. doi: 10.1152/jn.00607.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biju KC, Marks DR, Mast TG, Fadool DA. Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system. J Comp Neurol. 2008;506:161–179. doi: 10.1002/cne.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthoff M, Tschritter O, Berg D, Liepelt D, Schulte C, Machicao F, Haering HU, Fritsche A. Effect of genetic variation in Kv1. 3 on olfactory function Diabetes/Metabolism. Research and Reviews. 2009;25:523–527. doi: 10.1002/dmrr.979. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Koni PA, Wang P, Li G, Kaczmarek L, Wu Y, Li Y, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1. 3 regulates energy homeostasis and body weight. Hum Mol Genet. 2003;12:551–559. doi: 10.1093/hmg/ddg049. [DOI] [PubMed] [Google Scholar]
- 9.Tucker K, Overton JM, Fadool DA. Kv1. 3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int J Obes. 2008;32:1222–1232. doi: 10.1038/ijo.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niijima A, Nagai K. Effect of Olfactory Stimulation with Flavor of Grapefruit Oil and Lemon Oil on the Activity of Sympathetic Branch in the White Adipose Tissue of the Epididymis. Exp Biol Med. 2003;228:1190–1192. doi: 10.1177/153537020322801014. [DOI] [PubMed] [Google Scholar]
- 11.Shen J, Niijima A, Tanida M, Horii Y, Maeda K, Nagai K. Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neuroscience Letters. 2005;380:289–294. doi: 10.1016/j.neulet.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Niijima A, Tanida M, Horii Y, Maeda K, Nagai K. Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neuroscience Letters. 2005;383:188–193. doi: 10.1016/j.neulet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Tanida M, Shen J, Niijima A, Yamatodani A, Oishi K, Ishida N, Nagai K. Effects of olfactory stimulations with scents of grapefruit and lavender oils on renal sympathetic nerve and blood pressure in Clock mutant mice. Autonomic Neuroscience. 2008;139:1–8. doi: 10.1016/j.autneu.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Tanida M, Niijima A, Shen J, Nakamura T, Nagai K. Olfactory stimulation with scent of essential oil of grapefruit affects autonomic neurotransmission and blood pressure. Brain Research. 2005;1058:44–55. doi: 10.1016/j.brainres.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Tanida M, Shen J, Nakamura T, Niijima A, Nagai K. Day-night difference in thermoregulatory responses to olfactory stimulation. Neuroscience Letters. 2008;439:192–197. doi: 10.1016/j.neulet.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Tanida M, Niijima A, Shen J, Nakamura T, Nagai K. Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neuroscience Letters. 2006;398:155–160. doi: 10.1016/j.neulet.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 17.Koni PA, Khanna R, Chang MC, Tang MD, Kaczmarek LK, Schlichter LC, Flavella RA. Compensatory anion currents in Kv1. 3 channel-deficient thymocytes. J Biol Chem. 2003;278:39443–39451. doi: 10.1074/jbc.M304879200. [DOI] [PubMed] [Google Scholar]
- 18.Getchell TV, Liu H, Vaishnav RA, Kwong K, Stromberg AJ, Getchell ML. Temporal profiling of gene expression during neurogenesis and remodeling in the olfactory epithelium at short intervals after target ablation. J Neurosci Res. 2005;80:309–329. doi: 10.1002/jnr.20411. [DOI] [PubMed] [Google Scholar]
- 19.Rashotte ME, Basco PS, Henderson RP. Daily cycles in body temperature, metabolic rate, and substrate utilization in pigeons: influence of amount and timing of food consumption. Physiology & Behavior. 1995;57:731–746. doi: 10.1016/0031-9384(94)00315-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1459–R1467. doi: 10.1152/ajpregu.00612.2001. [DOI] [PubMed] [Google Scholar]
- 21.Bartholomew GA, Vleck D, Vleck CM. Instantaneous measurements of oxigen consumption during preflight warm up and postflight cooling in sphinged and saturniid moths. J Exp Biol. 1981;90:17–32. [Google Scholar]
- 22.Arch J, Hislop D, Wang S, Speakman J. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes. 2006:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- 23.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol (Lond) 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaxter K. Energy Metabolism in Animals and Man. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 25.Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neuroscience & Biobehavioral Reviews. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Hellweg R, Zueger M, Fink K, H÷rtnagl H, Gass P. Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiology of Disease. 2007;25:1–7. doi: 10.1016/j.nbd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone but not obesity-resistant rats. Behavioural Brain Research. 2007;180:190–196. doi: 10.1016/j.bbr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meguid MM, Koseki M, Yang ZJ, Gleason JR, Laviano A. Acute adaptive changes in food intake pattern following olfactory ablation in rats [Miscellaneous Article] Neuroreport. 1997;8:1439–1444. doi: 10.1097/00001756-199704140-00023. [DOI] [PubMed] [Google Scholar]
- 29.Marcilhac A, Maurel D, Anglade G, Ixart G, Mekaouche M, Héry F, Siaud P. Effects of Bilateral Olfactory Bulbectomy on Circadian Rhythms of ACTH, Corticosterone, Motor Activity and Body Temperature in Male Rats. Arch Physiol Biochem. 1997;105:552–559. doi: 10.1076/apab.105.6.552.3273. [DOI] [PubMed] [Google Scholar]
- 30.Vinkers CH, Breuer ME, Westphal KGC, Korte SM, Oosting RS, Olivier B, Groenink L. Olfactory bulbectomy induces rapid and stable changes in basal and stress-induced locomotor activity, heart rate and body temperature responses in the home cage. Neuroscience. 2009;159:39–46. doi: 10.1016/j.neuroscience.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Perret M, Aujard F, Seguy M, Schilling A. Olfactory Bulbectomy Modifies Photic Entrainment and Circadian Rhythms of Body Temperature and Locomotor Activity in a Nocturnal Primate. J Biol Rhythms. 2003;18:392–401. doi: 10.1177/0748730403254248. [DOI] [PubMed] [Google Scholar]
- 32.Saulea G, Hirscu M, Vidrascu N, Baciu I. Influence of bilateral olfactory bulbectomy on the circadian rhythm of phagocytic activity and phagocytic response in mice. Rom J Physiol. 1998;35:313–318. [PubMed] [Google Scholar]
- 33.Possidente B, Lumia AR, McGinnis MY, Teicher MH, deLemos E, Sterner L, Deros L. Olfactory bulb control of circadian activity rhythm in mice. Brain Research. 1990;513:325–328. doi: 10.1016/0006-8993(90)90475-q. [DOI] [PubMed] [Google Scholar]
- 34.Vagell M, McGinnis MY, Possidente B, Narasimhan V, Lumia AR. Olfactory bulbectomy increases basal suprachiasmatic cyclic AMP levels in male rats. Brain Res Bull. 1991;27:839–842. doi: 10.1016/0361-9230(91)90219-a. [DOI] [PubMed] [Google Scholar]
- 35.Jarosik J, Legutko B, Unsicker K, von Bohlen und Halbach O. Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Experimental Neurology. 2007;204:20–28. doi: 10.1016/j.expneurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Mucignat-Caretta C, Bondi M, Caretta A. Time course of alterations after olfactory bulbectomy in mice. Physiology & Behavior. 2006;89:637–643. doi: 10.1016/j.physbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Tucker K, Cavallin MA, Jean-Baptiste P, Biju KC, Overton JM, Pedarzani P, Fadool DA. The olfactory bulb: A metabolic sensor of brain insulin and glucose concentrations via a voltage-gated potassium channel. Results Probl Cell Differ. 2010;52:147–157. doi: 10.1007/978-3-642-14426-4_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julliard AK, Chaput MA, Apelbaum A, Aime P, Mahfouz M, Duchamp-Viret P. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behavioural Brain Research. 2007;183:123–129. doi: 10.1016/j.bbr.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 39.Prud'homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, Caillol M. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience. 2009;162:1287–1298. doi: 10.1016/j.neuroscience.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 40.Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- 41.Stephan F, Zucker I. Rat drinking rhythms: central visual pathways and endocrine factors mediating responsiveness to environmental illumination. Physiol Behav. 1972;8:315–326. doi: 10.1016/0031-9384(72)90379-4. [DOI] [PubMed] [Google Scholar]
- 42.Butler MP, Silver R. Basis of Robustness and Resilience in the Suprachiasmatic Nucleus: Individual Neurons Form Nodes in Circuits that Cycle Daily. J Biol Rhythms. 2009;24:340–352. doi: 10.1177/0748730409344800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruger M, Scheer F. Effects of circadian disruption on the cardiometabolic system. Reviews in Endocrine & Metabolic Disorders. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen J, Niijima A, Tanida M, Horii Y, Nakamura T, Nagai K. Mechanism of changes induced in plasma glycerol by scent stimulation with grapefruit and lavender essential oils. Neuroscience Letters. 2007;416:241–246. doi: 10.1016/j.neulet.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 45.Tschritter O, Machicao F, Stefan N, Schafer S, Weigert C, Staiger H, Spieth C, Haring HU, Fritsche A. A new variant in the human Kv1. 3 gene is associated with low insulin sensitivity and impaired glucose tolerance. J Clin Endocrinol Metab. 2006;91:654–658. doi: 10.1210/jc.2005-0725. [DOI] [PubMed] [Google Scholar]
- 46.Colley B, Tucker K, Fadool DA. Comparison of modulation of Kv1. 3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Receptors Channels. 2004;10:25–36. [PMC free article] [PubMed] [Google Scholar]
- 47.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1. 3) ion channel half-life and surface expression. Neuroscience. 2007;144:531–546. doi: 10.1016/j.neuroscience.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das P, Parsons AD, Scarborough J, Hoffman J, Wilson J, Thompson RN, Overton JM, Fadool DA. Electrophysiological and behavioral phenotype of insulin receptor defective mice. Physiology & Behavior. 2005;86:287–296. doi: 10.1016/j.physbeh.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadool DA, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1. 3. J Neurophysiol. 2000;83:2332–2348. doi: 10.1152/jn.2000.83.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542:413–429. doi: 10.1113/jphysiol.2002.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colley B, Cavallin M, Biju KC, Marks D, Fadool D. Brain-derived neurotrophic factor modulation of Kv1. 3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neuroscience. 2009;10:8. doi: 10.1186/1471-2202-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook KK, Fadool DA. Two adaptor proteins differentially modulate the phosphorylation and biophysics of Kv1. 3 ion channel by SRC kinase. J Biol Chem. 2002;277:13268–13280. doi: 10.1074/jbc.M108898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks DR, Fadool DA. Post-synaptic density perturbs insulin-induced Kv1. 3 channel modulation via a clustering mechanism involving the SH3 domain. J Neurochem. 2007;103:1608–1627. doi: 10.1111/j.1471-4159.2007.04870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Blood chemistry of intact Kv1.3 +/+ and −/− mice. Bar graph of fasting blood glucose (left), serum insulin (middle), and leptin (right) concentrations after 26 weeks of diet treatment. The lowercase letters indicate significant statistical differences within each fat pad as determined by a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test.
S2. Blood chemistry of sham and OBX surgically treated Kv1.3 +/+ and −/− mice. Bar graph of fasting blood glucose (left), serum insulin (middle), and leptin concentrations (right) after 26 weeks of diet treatment in sham- and OBX-treated mice. Lowercase letters indicate significant differences within +/+ mice and uppercase letters indicate significant differences within −/− mice as determined by a one-way analysis of variance (ANOVA; within a genotype) at the 95% confidence interval, followed by a Bonferroni post hoc test.
S3. Non-normalized metabolic data and analysis of covariance (ANCOVA). Non-normalized A) dark phase or activity-dependent metabolism and B) light phase or basal metabolism. Values represent mean ± SEM of 2 day averages within an animal, n=5. Scatter plot of metabolic rate versus weight are plotted for both genotypes maintained on the MHF-diet (C,E) = dark phase; (D,F) = light phase. Data were fit by linear regression and the elevation differences were compared by ANCOVA at the 95% confidence interval. Solid line and closed circles = sham MHF; Dashed line and open circles = OBX MHF. If a significant difference was detected in elevation, the p value (p) was reported. No significance is indicated by N.S., n=5. A-B) Lettering style, statistical analysis, and abbreviations as in Figure 2.
S4. Non-normalized total energy expenditure (TEE). A) Bar graphs of non-normalized TEE. Lettering, statistical analysis, and abbreviations as in Figure 2. All values represent mean ± SEM of 2 day averages within animal. Scatter plot of TEE versus weight of (B) +/+ and (C) −/− treated with a MHF-diet. Data were fit by linear regression and the elevation differences were compared by analysis of covariance (ANCOVA) at the 95% confidence interval. Solid line and closed circles = sham MHF; Dashed line and open circles = OBX MHF. If a significant difference was detected in elevation, the p value (p) was reported. No significance is indicated by N.S., n=5.