Abstract
The vertebrate heart is innervated by the sympathetic and parasympathetic components of the peripheral autonomic nervous system, which regulates its contractile rate and force. Understanding the mechanisms controlling sympathetic neuronal growth, differentiation, and innervation of the heart may provide insight into the etiology of cardiac arrhythmogenesis. In this review, we provide an overview of the cell signaling pathways and transcriptional effectors that regulate both the noradrenergic gene program during sympathetic neurogenesis and regional nerve density during cardiac innervation. We detail recent studies exploring transcriptional regulation of the bHLH transcription factor Hand1 in developing sympathetic neurons, and discuss how the Hand1 sympathetic neuron-specific cis-regulatory element may be further utilized to assess the contribution of altered sympathetic innervation to human cardiac disease.
Keywords: sympathetic nervous system, neural crest
Introduction
The peripheral autonomic nervous system innervates various organs, including the heart. It regulates two major aspects of cardiac function, heart rate and contractile force, through the opposing influences of sympathetic and parasympathetic efferent nerves. Sympathetic nerve fiber stimulation acutely increases both heart rate and contractile force, whereas parasympathetic nerve fiber stimulation diminishes heart rate and attenuates sympathetic effects on force. In addition to the beat-to-beat regulation of rate and force, a large number of experimental studies strongly suggest a role of cardiac sympathetic innervation in shaping the electrophysiological phenotype of the target tissue, via transcriptional regulation of genes encoding ion channel/transporter proteins and/or via their sustained post-translational modifications. Indeed, clinical studies have provided ample, yet largely circumstantial, evidence that spatially heterogeneous alterations in sympathetic nerve density in the diseased heart can give rise to increased susceptibility to life-threatening ventricular tachyarrhythmias. (20, 27, 37). Therefore, understanding the mechanisms by which these neurons grow, differentiate, and ultimately innervate the heart may be critical to understanding cardiac disease, specifically cardiac arrhythmogenesis.
Anatomy of cardiac sympathetic innervation
The sympathetic chain ganglia connect the heart and the central nervous system (12). Axons from preganglionic sympathetic neurons (abbreviated hereafter SNs) located in the intermediolateral column of the thoracic and lumbar spinal cord project to the sympathetic chain ganglia, also known as the paravertebral ganglia, which run bilaterally ventrolateral to the spinal column. The rostral-most of the sympathetic chain ganglia are termed the cervical ganglia. The majority of cardiac sympathetic nerves originate in postganglionic neurons located in the cervical and upper thoracic ganglia (also known as the stellate ganglia), mostly following the great arteries to join the cardiac plexus, located dorsal and caudal to the heart (21). In mammals, the majority of these neurons then project between the aorta and pulmonary trunk, following the paths of coronary arteries as they innervate the target regions. Sympathetic nerves run in epicardial bundles, and then individually dive into the myocardial wall, wherein they form neuroeffector junctions with their target cells. Intramural segments of postganglionic sympathetic axons exhibit periodic swellings, or ‘varicosities’, which correspond to the neurotransmitter release sites. The density of axonal projections from postganglionic SNs in the heart exhibits marked spatial variability. Axonal density is high in the sinus node, and displays epicardial to endocardial gradients in the right and left ventricular free wall. Intriguingly, collapse of this transmural innervation gradient in the adult mouse is accompanied by a reversal of the intrinsic transmural repolarization gradient, giving rise to increased arrhythmia susceptibility (20). The latter finding further supports the notion that changes in regional density of sympathetic nerves exert long-term adverse effects on the electrical phenotype of the target tissue. Or in more general terms, maintenance of physiological innervation gradients in the heart is a necessary requirement to sustain physiological repolarization gradients across the chamber walls. Therefore, understanding the mechanisms controlling regional nerve density is critical in understanding cardiac arrhythmogenesis.
Development of the sympathetic nervous system
The process of sympathetic neurogenesis has been extensively studied (1, 3, 8, 15–17, 36). SNs are derived from neural crest cells, which arise from the margin of the neuroepithelium, and delaminate and migrate throughout the developing embryo. Neural crest cells can differentiate into both neural and non-neural tissue, and their lineage is restricted by the axial level at which they originate. Cardiac neural crest, which originate immediately caudal to the otic placode, at the level of somites 1 – 3, differentiate into the smooth muscle cells of the great arteries and the connective tissue that forms the aorticopulmonary and membranous septa. Postganglionic sympathetic neurons, along with a variety of other components of the peripheral nervous system, are derived from the trunk neural crest, which originates caudally to somite 4 (23).
SNs are catecholaminergic, in other words, they use norepinephrine as their main neurotransmitter. This distinguishes postganglionic SNs from parasympathetic neurons, which are solely cholinergic neurons, and use acetylcholine as a neurotransmitter. To synthesize norepinephrine from its precursor, L-tyrosine, SNs must produce three enzymes, namely, tyrosine hydroxylase (TH), dopamine β-hydroxylase (DBH), and phenylethanolamine N-methyl transferase (PNMT). Although the potency of SN progenitor is restricted based upon the axial level at which they originate, upregulation of these specific biosynthetic enzymes is driven by local signaling cues proximal to the dorsal aorta (42). Secreted signaling molecules termed Bone morphogenetic proteins (Bmps), specifically Bmp2, Bmp4, and Bmp7, derived from the smooth muscle cells of the dorsal aorta, are sufficient to induce neural crest cells to upregulate SN differentiation markers, including TH, as shown in both explant cultures and chick embryos (35, 41, 44, 45). Conversely, exogenous application of a Bmp antagonist, noggin, prevents SN differentiation (40). Conditional ablation of Bmpr1a, a Bmp receptor, in mouse neural crest cells causes massive SN cell death following migration, whereas ablation of Smad4, the transcriptional effector of canonical Bmp signaling, causes diminished TH expression and proliferation, but does not otherwise appreciably affect sympathetic neurogenesis (2, 30) suggesting that BMPs function through other, potentially protein kinase A-mediated, mechanisms (25) to regulate sympathetic neurogenesis.
The major transcription factors that have been shown to regulate sympathetic neurogenesis, namely the paired-like homeodomain transcription factor Phox2b, the bHLH transcription factors Ascl1 and Hand2, and the Zn- nger transcription factors Gata2, Gata3, and Insm1, all act downstream of Bmp signaling. Phox2b is a master regulator of sympathetic neurogenesis. Loss of Phox2b function in mice causes catastrophic cell death in all developing autonomic neurons and a failure to either upregulate or maintain Ascl1, Hand2, Gata2, Gata3, and Insm1 expression (32, 46). These factors all contribute to establishing proper SN cell number by promoting either cell proliferation or survival. Rather than regulate specific noradrenergic differentiation programs, Ascl1 and Insm1 are thought to regulate the timing of noradrenergic differentiation, as both knockout models display delayed expression of TH and DBH (33, 46).
Indeed, like many neuronal cell types, SN differentiation transcriptionally conforms to a common basic developmental program in which basic-helix-loop-helix (bHLH) transcription factors cooperate with homeodomain transcription factors to effect neuronal progenitor specification (15, 20). Phox2b cooperates with one of the factors it regulates, Hand2 (14), and these two factors then drive neuronal precursor proliferation and noradrenergic differentiation, for example, by regulating expression of the norepinephrine biosynthetic enzyme DBH (32, 38, 47), and Hand2-related bHLH factor Hand1 (29); Vincentz et al., 2012, J Neurosci., in press). Loss of Hand2 gene function has been shown to disrupt SN in both fish and mice (10, 13, 26, 29), and gain of Hand2 function in chick directs neural crest cells and parasympathetic ganglia to adopt noradrenergic characteristics, such as TH and DBH expression (13, 14, 31). Indeed, Phox2b and Hand2, along with Gata3, are thought to be the main effectors of the noradrenergic gene program.
Following their proliferation and differentiation, SNs must properly innervate the heart to regulate cardiomyocyte function. Aberrant sympathetic innervation may trigger lethal arrhythmias (4, 6, 34). Explanted hearts of cardiac transplant recipients with a clinical history of ventricular tachyarrhythmia display increased cardiac nerve density, as assessed by immunohistochemical analysis (4), compared to hearts from patients without arrhythnmia anamnesis. A neurotrophin, nerve growth factor (NGF), and a Class 3 secreted semaphorin, Sema3a, have been implicated in the proper patterning of cardiac sympathetic nerves during development and in diseased hearts. NGF expression is associated with tissues displaying a high innervation density (11), including the heart. Targeted ablation of Edn1, which regulates NGF expression, diminishes cardiac sympathetic innervation and norepinephrine concentration (18), phenotypes that are partially rescued by cardiac-restricted transgenic overexpression of NGF (9, 18). Sema3a-deficient mice display abnormalities of both the nerves that project from the cervical ganglia into the heart and those that innervate the subepicardium and subendocardium, causing sinus bradycardia and abrupt sinus arrest (20). Transgenic overexpression of Sema3a specifically in the heart via the α-myosin heavy chain promoter caused a reduction in overall sympathetic nerve density and a collapse of the transmural innervation gradient, increasing susceptibility to induced ventricular tachyarrhythmias (19). These genetic models illustrate both the critical role of tighly regulated cardiac sympathetic innervation in maintaining cardiac performance, and also the contribution of adverse sympathetic remodeling to cardiac disease. The plasticity of these cardiac nerves, as evinced by alterations in their innervation patterns following myocardial infarction, or in hypertrophic hearts (22) begs the question: do cardiac innervation patterns also change with ageing, and if so, do these changes occur due to a loss of cardiac innervation, a loss of postganglionic SNs innervating the heart, or both?
Postganglionic sympethetic neurons require maintenance cues following differentiation
Hand2 disruption in postmitotic, differentiated SNs, either via siRNA knockdown in chick, or through conditional gene ablation in mice, causes reduced ganglionic cell number and a failure to maintain noradrenergic characteristics (39). Additionally, analyses of Hand2 hypomorphic mutants show that, as proposed in previous studies (10, 13), transcriptional programs in the developing SNs are sensitive to Hand2 gene dosage (Vincentz et al., 2012, J Neurosci., in press). Together, these data indicate that the SNs require continual Hand2 transcriptional input at a critical threshold to maintain proper cell numbers and normal gene expression. Additionally, it should be noted that although loss of Gata3 function causes reduced TH and DBH expression (24), this reduced gene expression seems to reflect a progressive loss of expression, rather than a failure to upregulate noradrenergic programs (43). This suggests that Gata3 may also be required for the maintenance of expression of genes responsible for a noradrenergic phenotype, rather than its initial upregulation.
Regulation of Hand1 expression in postganglionic sympathetic neurons
As mentioned previously, a downstream target of Hand2 during sympathetic neurogenesis is the related factor, Hand1. Although siRNA knockdown experiments in cultured mouse superior cervical ganglia, a subtype of SNs which express both Hand1 and Hand2, suggests that these factors perform overlapping functions, such as the promotion of cell survival (7), evidence from genetic interaction studies suggest that Hand1 does not significantly contribute to the noradrenergic gene program in these cells (Vincentz et al., 2012, J Neurosci., in press). Gene expression in the SNs is sensitive to Hand2 gene dosage; however, TH and DBH protein expression within these cells is unperturbed by a loss of Hand1 function, either alone or coupled with a Hand2 heterozygous background (Vincentz et al., 2012, J Neurosci., in press). Although its functional role in SN development is likely minimal, Hand1 in the SNs is worthy of study, as understanding the mechanisms by which it is transcriptionally regulated provides insight into the SN developmental program.
Hand1 is expressed solely in SNs and no other neuronal subtype (5, 28). Recent studies have defined an evolutionarily conserved Hand1 cis-regulatory element, to which we will heretofore refer as Hand1SG, sufficient to drive lacZ reporter gene expression throughout the developing and postnatal SNs (Figure 1A; Vincentz et al., 2012, J Neurosci., in press). Hand2 and Phox2b have been shown, through chromatin immunoprecipitation and electro-mobility shift assays, to bind evolutionarily conserved consensus-binding sites within this SN-specific enhancer. Mutation of either the Phox2 binding site, to which Phox2b binds, or the three E-box binding sites, bound by Hand2, abolishes reporter gene expression in transient transgenic embryos. Similarly, disruption of Hand2 function attenuates Hand1SG-lacZ reporter gene expression in a dosage-dependent manner. These data sets confirm that Hand2 is a direct upstream regulator of Hand1 transcription. Given that Hand2 and Hand1 are thought to perform overlapping functions, it is surprising that, in the face of diminished Hand2 function, Hand1 cannot feed back to auto-regulate its own expression. Although Hand2 and Hand1 can behave similarly in SNs, for example, they both interact with Phox2b identically in vitro, Hand1 and Hand2 display different binding affinities for the conserved E-boxes within the Hand1SG enhancer. This difference in binding affinity suggests that these two factors are not functionally identical, and both potentially accounts for the inability of Hand1 to auto-regulate and raises the possibility that Hand1 may fulfill other unique roles within the SN gene program.
Figure 1. The Hand1 sympathetic neuron-specific enhancer drives reporter gene expression in neurons innervating the heart.
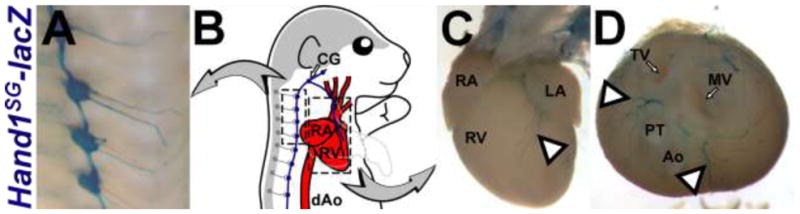
A) X-gal stained sympathetic ganglia in P0 Hand1SG-lacZ(+) mice. B) An illustration demonstrates how the CNS (gray) and the heart (red) are linked by the Hand1SG-lacZ–expressing sympathetic neurons (blue). Boxes highlight the stained tissues shown in A, C, and D. C, D) X-gal stained sympathetic nerves in P0 Hand1SG-lacZ(+) mice. In D, the atria and great vessels have been removed to provide an unobstructed view of the nerves that project from between the atrioventricular canal and outflow tract (arrowheads). Ao – aorta, CG – cervical ganglia, dAo – descending aorta, LA – left atrium, MV – mitral valve, PT – pulmonary trunk, RA – right atrium, RV – right ventricle, TV – tricuspid valve.
An intriguing faculty of this reporter is its ability to clearly label neurons innervating the heart (Figure 1C, D). Given the endogenous expression profile of Hand1, and the observation that the Hand1SG-lacZ transgene is robustly expressed in the sympathetic chain ganglia, it is likely that these are sympathetic nerve fibers, although further expression analyses are required to confirm this hypothesis, and to assess the extent of SN nerve fiber labeling.
As the Hand1SG enhancer has been shown to be a sensitive and tissue-specific in vivo reporter of at least two essential transcriptional effectors of the noradrenergic program, it also potentially has broad applications for visualizing SNs in developmental and disease contexts. For example, previous studies have definitively demonstrated that the Hand1SG-lacZ reporter is specifically sensitive to Hand2 gene dosage (Vincentz et al., 2012, J Neurosci., in press). Furthermore, continual Hand2 transcriptional input is required to maintain noradrenergic gene expression in differentiated SNs (39). Thus, the Hand1SG-lacZ transgenic reporter would provide a useful tool with which to assess the role of Hand2 in the maintenance of adult SN function, for example, whether Hand2 function attenuates with age, or whether Hand2 function is altered in the SN following myocardial infarction.
Given its robust and tissue-specific expression, the Hand1SG enhancer could also be used to generate a tamoxifen-inducible Cre recombinase transgene. This Cre driver could then be employed to specifically ablate gene function in SNs of the adult animal. This would bypass the pre- or perinatal lethality that typically occurs when factors critical to the noradrenergic gene program are ablated in SNs or their progenitors. Thus, the Hand1SG enhancer, utilized in conjunction with other emerging molecular tools, shows promise to provide profound insight into the contribution of altered sympathetic innervation to human cardiac disease.
Acknowledgments
We thank Danny Carney for technical assistance. We thank the Herman B Wells Center Cardiac Developmental Biology Group for helpful discussions. Infrastructural support at the Herman B Wells Center is partially supported by the Riley Children’s Foundation and Division of Pediatric Cardiology. Grant support for this work was provided by: 1P01HL085098-05 (ABF)
Footnotes
Conflict of Interest: None
References
- 1.Apostolova G, Dechant G. Development of neurotransmitter phenotypes in sympathetic neurons. Autonomic neuroscience: basic & clinical. 2009;151:30–38. doi: 10.1016/j.autneu.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Buchmann-Moller S, Miescher I, John N, Krishnan J, Deng CX, Sommer L. Multiple lineage-specific roles of Smad4 during neural crest development. Developmental biology. 2009;330:329–338. doi: 10.1016/j.ydbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Cane KN, Anderson CR. Generating diversity: Mechanisms regulating the differentiation of autonomic neuron phenotypes. Autonomic neuroscience: basic & clinical. 2009;151:17–29. doi: 10.1016/j.autneu.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 5.Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- 6.Dae MW, Lee RJ, Ursell PC, Chin MC, Stillson CA, Moise NS. Heterogeneous sympathetic innervation in German shepherd dogs with inherited ventricular arrhythmia and sudden cardiac death. Circulation. 1997;96:1337–1342. doi: 10.1161/01.cir.96.4.1337. [DOI] [PubMed] [Google Scholar]
- 7.Doxakis E, Howard L, Rohrer H, Davies AM. HAND transcription factors are required for neonatal sympathetic neuron survival. EMBO reports. 2008;9:1041–1047. doi: 10.1038/embor.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annual review of neuroscience. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 9.Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Developmental biology. 1995;169:309–321. doi: 10.1006/dbio.1995.1146. [DOI] [PubMed] [Google Scholar]
- 10.Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Developmental biology. 2008;319:179–191. doi: 10.1016/j.ydbio.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heumann R, Korsching S, Scott J, Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. The EMBO journal. 1984;3:3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildreth V, Anderson RH, Henderson DJ. Autonomic innervation of the developing heart: origins and function. Clinical anatomy. 2009;22:36–46. doi: 10.1002/ca.20695. [DOI] [PubMed] [Google Scholar]
- 13.Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Developmental biology. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- 14.Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–4081. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- 15.Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Developmental biology. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Developmental biology. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Huber K, Kalcheim C, Unsicker K. The development of the chromaffin cell lineage from the neural crest. Autonomic neuroscience: basic & clinical. 2009;151:10–16. doi: 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Ieda M, Fukuda K, Hisaka Y, Kimura K, Kawaguchi H, Fujita J, Shimoda K, Takeshita E, Okano H, Kurihara Y, Kurihara H, Ishida J, Fukamizu A, Federoff HJ, Ogawa S. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. The Journal of clinical investigation. 2004;113:876–884. doi: 10.1172/JCI19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nature medicine. 2007;13:604–612. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- 20.Ieda M, Fukuda K. Cardiac innervation and sudden cardiac death. Current cardiology reviews. 2009;5:289–295. doi: 10.2174/157340309789317904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anatomy and embryology. 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Ieda M, Kanazawa H, Yagi T, Tsunoda M, Ninomiya S, Kurosawa H, Yoshimi K, Mochizuki H, Yamazaki K, Ogawa S, Fukuda K. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy. Circulation research. 2007;100:1755–1764. doi: 10.1161/01.RES.0000269828.62250.ab. [DOI] [PubMed] [Google Scholar]
- 23.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 24.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Margiotta JF, Howard MJ. BMP4 supports noradrenergic differentiation by a PKA-dependent mechanism. Dev Biol. 2005;286:521–536. doi: 10.1016/j.ydbio.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- 27.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiological reviews. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa Y, Cserjesi P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development. 2004;131:2195–2204. doi: 10.1242/dev.01091. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–126. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009;136:3575–3584. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller F, Rohrer H. Molecular control of ciliary neuron development: BMPs and downstream transcriptional control in the parasympathetic lineage. Development. 2002;129:5707–5717. doi: 10.1242/dev.00165. [DOI] [PubMed] [Google Scholar]
- 32.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 33.Pattyn A, Guillemot F, Brunet JF. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Developmental biology. 2006;295:67–75. doi: 10.1016/j.ydbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Qu Z. Nonlinear dynamic control of irregular cardiac rhythms. Journal of cardiovascular electrophysiology. 2004;15:1186–1187. doi: 10.1046/j.1540-8167.2004.04484.x. [DOI] [PubMed] [Google Scholar]
- 35.Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- 36.Rohrer H. Transcriptional control of differentiation and neurogenesis in autonomic ganglia. The European journal of neuroscience. 2011;34:1563–1573. doi: 10.1111/j.1460-9568.2011.07860.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. The Journal of clinical investigation. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rychlik JL, Gerbasi V, Lewis EJ. The interaction between dHAND and Arix at the dopamine beta-hydroxylase promoter region is independent of direct dHAND binding to DNA. J Biol Chem. 2003;278:49652–49660. doi: 10.1074/jbc.M308577200. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Lin S, Pape M, Ernsberger U, Stanke M, Kobayashi K, Howard MJ, Rohrer H. The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Developmental biology. 2009;329:191–200. doi: 10.1016/j.ydbio.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999;24:861–870. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 41.Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 42.Stern CD, Artinger KB, Bronner-Fraser M. Tissue interactions affecting the migration and differentiation of neural crest cells in the chick embryo. Development. 1991;113:207–216. doi: 10.1242/dev.113.1.207. [DOI] [PubMed] [Google Scholar]
- 43.Tsarovina K, Pattyn A, Stubbusch J, Muller F, van der Wees J, Schneider C, Brunet JF, Rohrer H. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 44.Varley JE, Wehby RG, Rueger DC, Maxwell GD. Number of adrenergic and islet-1 immunoreactive cells is increased in avian trunk neural crest cultures in the presence of human recombinant osteogenic protein-1. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203:434–447. doi: 10.1002/aja.1002030406. [DOI] [PubMed] [Google Scholar]
- 45.Varley JE, Maxwell GD. BMP-2 and BMP-4, but not BMP-6, increase the number of adrenergic cells which develop in quail trunk neural crest cultures. Experimental neurology. 1996;140:84–94. doi: 10.1006/exnr.1996.0118. [DOI] [PubMed] [Google Scholar]
- 46.Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–481. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Dev Biol. 2003;262:183–193. doi: 10.1016/s0012-1606(03)00361-0. [DOI] [PubMed] [Google Scholar]


