Abstract
In temperate regions, seasonal epidemics of many mosquito-borne viruses are triggered when mosquito populations shift from feeding on avian to mammalian hosts. We investigated effects of temperature on the timing of bird-to-mammal shifts using an 8 year dataset of blood-meals from a mosquito (Culex erraticus) in Alabama, USA. As expected, Cx. erraticus shifted from avian to mammalian hosts each year. The timing of the shift, however, varied considerably among years. Harshness of the preceding winter (chill accumulation) explained 93 per cent of the variation in the timing of bird-to-mammal shifts, with shifts occurring later in years following harsher winters. We hypothesize that winter temperatures drive the timing of bird-to-mammal shifts through effects on host reproductive phenology. Because mosquitoes target birds during the nesting season, and bird nesting occurs later in years following colder winters, later nesting dates result in a concomitant delay in the timing of bird-to-mammal host shifts. Global increases in winter temperatures could cause significant changes in the timing of seasonal host shifts by mosquitoes, with prolonged periods of epidemic transmission of mosquito-borne diseases.
Keywords: climate, mosquito, host shift, arbovirus
1. Introduction
For some of the most pathogenic mosquito-borne viruses, including West Nile virus (WNV) and eastern equine encephalomyelitis (EEE) virus, birds are the primary reservoir hosts [1]. Mosquitoes acquire these viruses from infected birds during blood feeding, and then transmit virus to humans and other susceptible animals in subsequent feedings. The mosquito species that transmit WNV and EEE to humans feed on both birds and mammals. These ‘bridge vectors’ feed predominantly on birds in the spring and early summer, but feed predominantly on mammals later in the year [2]. This seasonal shift in host use by mosquitoes drives the timing and intensity of epidemics of mosquito-borne viruses, such as WNV [3]. Therefore, elucidating the mechanisms that drive the timing of bird-to-mammal shifts by mosquitoes is critical for predicting the phenology of epidemics of mosquito-borne pathogens. To this end, we examined annual variation in the timing of bird-to-mammal shifts by analysing an 8 year dataset of blood-meal identifications of field-collected females of Culex erraticus (Dyar and Knab), a mosquito implicated in the transmission of EEE virus [4,5] that feeds mainly on birds and mammals [4,6]. We used regression analysis to explore the relationship between temperature patterns and when shifts occurred. Temperature variables were chosen through discussions with phenology ecologists, who invoked the importance of winter cold (chill accumulation) and spring warming (growing degree units) on the timing of biological events, such as budburst in temperate trees [7] and crop plants [8], and the spring emergence of pollinating insects [9]. In addition, studies investigating climate effects on mosquito abundance have found that mosquito abundance in spring and summer are not linked to weather of individual months or even seasons, but to a chain of climate events that begin as early as the preceding autumn [10].
2. Material and methods
We investigated year-to-year variation in the timing of bird-to-mammal shifts through identification of blood meals from field-collected mosquitoes, from May through to August during 8 years (2001–2004 and 2006–2009). Blood-engorged female mosquitoes were aspirated weekly from resting sites [11] scattered throughout a mosaic of forest and wetland in Tuskegee National Forest, Alabama (AL), USA [4,12]. All engorged females were processed individually for blood-meal analysis via PCR, following published methods [12,13]. The timing of the bird-to-mammal shift was estimated based on the observed point in which the monthly proportion of blood meals from birds crossed the 50 per cent threshold (figure 1). The relationship between the timing of host shifts and annual temperature regimes was investigated using stepwise multiple regression analysis. We selected (a priori) three temperature variables (winter harshness, spring warming and average yearly air temperature) that are commonly used as independent variables in modelling studies to predict biological phenomena. Year was included in the analysis to account for unexplained variability among years. Variance inflation factors were calculated to check multi-collinearity of independent variables.
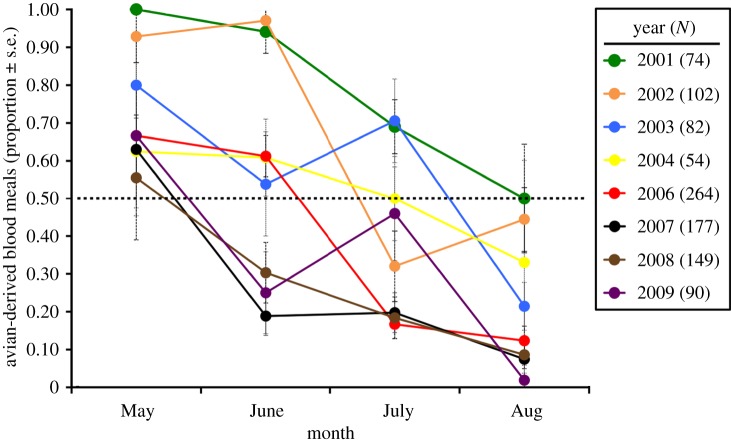
Figure 1.
Year to year variation in bird-to-mammal host shifts by Culex erraticus. The dashed line represents 50 : 50 feeding on birds and mammals. N represents the total mosquito bloodmeals identified each year.
Chill accumulation was calculated as the number of hours below 7.2°C [14] for the period between October and March (representing 96.3 ± 1.5% of the total chill hours each year). Growing degree units were calculated by taking the average of the daily maximum and minimum temperatures compared with a base temperature (10°C) [15] starting on 1 January [16] and ending on 30 April (prior to mosquito collection months). Temperature data were from E. V. Smith Research Center, AL (Auburn University), approximately 20 km from the site of mosquito collections. Data is available upon request.
3. Results
Overall, herons (great blue and yellow-crowned night heron) and white-tailed deer were the most commonly used avian and mammalian hosts, respectively. Together these three host species accounted for 707 (71.3%) of the 992 Cx. erraticus blood meals identified. More than 60 other host species were also identified from blood-fed Cx. erraticus, reported elsewhere [6,17].
Temperature patterns were quite variable from year to year. Winter harshness ranged from just 1172 h below 7.2°C in 2008 to 1590 h below 7.2°C in 2001 (a difference of 30.3% between these two years). Spring warming (January to April) was greatest in 2007, and lowest in 2004, with 911 and 695 growing degree-days during 2007 and 2004, respectively (a difference of 26.9%). Average annual temperature was highest in 2007 (18.94°C) and lowest in 2003 (17.28°C). Variance inflation factors were between 2.67 and 2.74, well within acceptable levels.
Culex erraticus demonstrated a notable shift in host use in each year, feeding primarily on birds in May of each year, and then shifting to feeding primarily on mammals by August (figure 1). The timing of the shift varied considerably between years. In some years (2007–2009), the shift occurred early in the season (May or June), whereas in other years (2001, 2003), the shift occurred as late as August. The timing of the shift was strongly positively associated with winter harshness (figure 2; r2 = 0.93, F = 80.55, p = 0.0001), such that each additional winter hour below 7.2°C is associated with a delay in bird-to-mammal shift by 0.22 (±0.02) days. No other variables were significant at α = 0.05.
Figure 2.
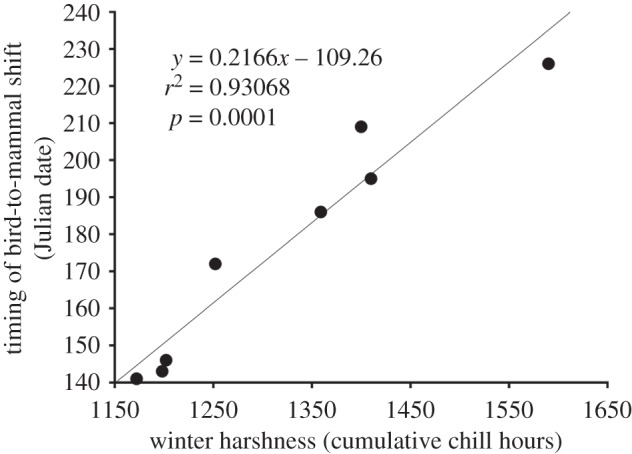
Relationship between winter harshness and the timing of host shifts by mosquitoes.
4. Discussion
Shifts from avian to mammalian hosts by mosquitoes are an important part of the epidemiology of mosquito-borne zoonotic viruses, and are thought to drive the timing and intensity of annual outbreaks in humans [3]. We demonstrate that the timing of bird-to-mammal shifts can vary considerably from one year to the next and that later shifts are preceded by colder winters. We hypothesize that colder winters may affect the timing of bird-to-mammal shifts by causing a delay in the breeding period of avian hosts. Because seasonal patterns of host use by mosquitoes track the breeding phenology of their hosts [6], temporal shifts in breeding periods of birds should result in later shifts to non-avian hosts. During their nesting period, nestling and brooding birds are more susceptible to attacking mosquitoes, owing to a lack of both defensive plumage (nestlings), and inability to use defensive movements (mothers and nestlings) [18], resulting in proportionally more blood meals being taken from birds during the nesting period. As young birds fledge, both fledglings and parent birds are better able to defend themselves against attacking mosquitoes, and the proportion of blood meals taken from birds decreases. The decrease in feeding on birds corresponds to a concomitant increase in mosquitoes feeding on mammals [6].
The timing of breeding in birds is overwhelmingly driven by energy demands of the mother birds [19]. Herons, the main bird hosts in our study, are no exception [20]. The earlier in the year that a female bird lays, the higher the probability that nestlings will survive and successfully fledge [19,21], so females lay eggs as soon as they are physiologically capable. Energy thresholds determine when herons are able to lay their first clutch of eggs [22]. Extended periods of low temperatures affect a female's ability to reach this minimum energy threshold, because the energy stores that a female would normally use to produce eggs are depleted in colder winters, thereby delaying when the energy threshold is reached. Low temperatures also affect the availability of food (prey items), exacerbating the energy deficit that the birds experience during colder winters [22]. The main prey items of herons (aquatic vertebrates and invertebrates) [23] are less active and experience higher mortalities during colder winters [24–26], thereby reducing their availability to foraging birds.
We demonstrate here, for the first time to our knowledge, that the timing of bird-to-mammal shifts by mosquitoes varies considerably between years and is linked to harshness of the preceding winter. We propose that harsh winters result in delayed bird nesting dates, and therefore, the temporal window of mosquitoes feeding on avian hosts is extended to later in the year. Because host shifts drive epidemics of mosquito-borne viruses, climate change, especially warmer winters, could result in earlier-than-normal host shifts that could, in turn, drive prolonged periods of epidemic transmission of mosquito-borne viruses.
Acknowledgements
Thanks to Dr Russell Wright and Dr Jeff Sibley for insightful discussions regarding temperature and phenology. Sean Graham and Christina Schmidt offered helpful comments on early drafts of the manuscript. AWIS Weather Services generously provided temperature data. This work was supported by NIAID, project no. R01AI049724.
References
- 1.Weaver S. C., Barrett A. D. 2004. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2, 789–801 10.1038/nrmicro1006 (doi:10.1038/nrmicro1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edman J. D., Taylor D. J. 1968. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science 161, 67–68 10.1126/science.161.3836.67 (doi:10.1126/science.161.3836.67) [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick A. M., Kramer L. D., Jones M. J., Marra P. P., Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, e82. 10.1371/journal.pbio.0040082 (doi:10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupp E. W., Klingler K., Hassan H. K., Viguers L. M., Unnasch T. R. 2003. Transmission of eastern equine encephalomyelitis virus in central Alabama. Am. J. Trop. Med. Hyg. 68, 495–500 [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S. B., Lewoczko K., Huddleston D. B., Moody E., Mukherjee S., Dunn J. R., Jones T. F., Wilson R., Moncayo A. C. 2009. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am. J. Trop. Med. Hyg. 81, 452–456 [PubMed] [Google Scholar]
- 6.Burkett-Cadena N. D., et al. 2011. Host reproductive phenology drives seasonal patterns of host use in mosquitoes. PLoS ONE 6, e17681. 10.1371/journal.pone.0017681 (doi:10.1371/journal.pone.0017681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter A. F., Lechowicz M. J. 1992. Predicting the timing of budburst in temperate trees. J. Appl. Ecol. 29, 597–604 10.2307/2404467 (doi:10.2307/2404467) [DOI] [Google Scholar]
- 8.Cesaraccio C., Spano D., Snyder R. L., Duce P. 2004. Chilling and forcing model to predict bud burst of crops and forest species. Agr. For. Meteorol. 126, 1–3 10.1016/j.agrformet.2004.03.002 (doi:10.1016/j.agrformet.2004.03.002) [DOI] [Google Scholar]
- 9.Kraemer M. E., Favi F. D. 2010. Emergence phenology of Osmia lignaria subsp. lignaria (Hymenoptera: Megachilidae), its parasitoid Chrysura kyrae (Hymenoptera: Chrysididae), and bloom of Cercis canadensis. Environ. Entomol. 39, 351–358 10.1603/en09242 (doi:10.1603/en09242) [DOI] [PubMed] [Google Scholar]
- 10.Reisen W. K., Cayan D., Tyree M., Barker C. M., Eldridge B., Dettinger M. 2008. Impact of climate variation on mosquito abundance in California. J. Vector Ecol. 33, 89–98 10.3376/1081-1710(2008)33[89:IOCVOM]2.0.CO;2 (doi:10.3376/1081-1710(2008)33[89:IOCVOM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Burkett-Cadena N. D., Eubanks M. D., Unnasch T. R. 2008. Preference of female mosquitoes for natural and artificial resting sites. J. Am. Mosq. Control Assoc. 24, 228–235 10.2987/5662.1 (doi:10.2987/5662.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan H. K., Cupp E. W., Hill G. E., Katholi C. R., Klingler K., Unnasch T. R. 2003. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 69, 641–647 [PubMed] [Google Scholar]
- 13.Lee J. H., Hassan H. K., Hill G. E., Cupp E. W., Higazi T. B., Mitchell C. J., Godsey M. S., Jr, Unnasch T. R. 2002. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am. J. Trop. Med. Hyg. 66, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunley R. J., Atkinson C. J., Jones H. G. 2006. Chill unit models and recent changes in the occurrence of winter chill and spring frost in the United Kingdom. J. Hort. Sci. Biotech. 81, 949–958 [Google Scholar]
- 15.McMaster G. S., Wilhelm W. W. 1997. Growing degree-days: one equation, two interpretations. Agr. For. Meteorol. 87, 291–300 10.1016/S0168-1923(97) (doi:10.1016/S0168-1923(97)) [DOI] [Google Scholar]
- 16.Stewart C. D., Braman S. K., Sparks B. L., Williams-Woodward J. L., Waded G. L., Latimere J. G. 2002. Comparing an IPM pilot program to a traditional cover spray program in commercial landscapes. J. Econ. Entomol. 95, 789–796 10.1603/0022-0493-95.4.789 (doi:10.1603/0022-0493-95.4.789) [DOI] [PubMed] [Google Scholar]
- 17.Estep L. K., McClure C. J. W., Burkett-Cadena N. D., Hassan H. K., Hicks T. L., Unnasch T. R., Hill G. E. 2011. A multi-year study of mosquito feeding patterns on avian hosts in a southeastern focus of eastern equine encephalitis virus. Am. J. Trop. Med. Hyg. 84, 718–726 10.4269/ajtmh.2011.10-0586 (doi:10.4269/ajtmh.2011.10-0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkett-Cadena N. D., Ligon R. A., Liu M., Hassan H. K., Hill G. E., Eubanks M. D., Unnasch T. R. 2010. Vector–host interactions in avian nests: do mosquitoes prefer nestlings over adults? Am. J. Trop. Med. Hyg. 83, 395–399 10.4269/ajtmh.10-0048 (doi:10.4269/ajtmh.10-0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrins C. M. 1965. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647 10.2307/2453 (doi:10.2307/2453) [DOI] [Google Scholar]
- 20.Butler R. W. 1993. Time of breeding in relation to food availability of female great blue herons (Ardea herodias). Auk 110, 693–701 [Google Scholar]
- 21.Perrins C. M. 1970. The timing of birds’ breeding seasons. Ibis 112, 242–255 10.1111/j.1474-919X.1970.tb00096.x (doi:10.1111/j.1474-919X.1970.tb00096.x) [DOI] [Google Scholar]
- 22.Murton R. K., Westwood N. J. 1977. Avian breeding cycles. Oxford, UK: Clarendon Press [Google Scholar]
- 23.Riegner M. F. 1982. The diet of yellow-crowned night-herons in the eastern and southern United States. Colonial Waterbirds 5, 173–176 10.2307/1521050 (doi:10.2307/1521050) [DOI] [Google Scholar]
- 24.Bauer L. J., Miller T. J. 2010. Spatial and interannual variability in winter mortality of the blue crab (Callinectes sapidus) in the Chesapeake Bay. Estuaries Coasts 33, 678–687 10.1007/s12237-009-9237-x (doi:10.1007/s12237-009-9237-x) [DOI] [Google Scholar]
- 25.Fullerton A. H., Garvey J. E., Wright R. A., Stein R. A. 2000. Overwinter growth and survival of largemouth bass: interactions among size, food, origin, and winter severity. Trans. Am. Fisheries Soc. 129, 1–12 (doi:10.1577/1548-8659(2000)129<0001:OGASOL>2.0.CO;2) [DOI] [Google Scholar]
- 26.Hasler C. T., Suski C. D., Hanson K. C., Cooke S. J., Philipp D. P., Tufts B. L. 2009. Effect of water temperature on laboratory swimming performance and natural activity levels of adult largemouth bass. Can. J. Zool. 87, 589–596 10.1139/Z09-044 (doi:10.1139/Z09-044) [DOI] [Google Scholar]