Abstract
The social insect soldier is perhaps the most widely known caste, because it often exhibits spectacular weapons, such as highly enlarged jaws or reinforced appendages, which are used to defend the colony against enemies ranging in size from wasps to anteaters. We examined the function of the enlarged forelimbs of soldiers (both male and female) of the eusocial, gall-inhabiting insect Kladothrips intermedius, and discovered that they have little impact on their ability to repel the specialized invading thrips Koptothrips species. While the efficacy of the enlarged forelimb appears equivocal, we show that soldiers secrete strong antifungal compounds capable of controlling the specialized insect fungal pathogen, Cordyceps bassiana. Our data suggest that these thrips soldiers have evolved in response to selection by both macro- and micro-organisms. While it is unknown whether specialized fungal pathogens have been major selective agents in the evolution of the soldier caste in general, they were probably present when sociality first evolved and may have been the primordial enemies of social insects.
Keywords: social insects, soldiers, thrips, morphometrics, antimicrobials, caste
1. Introduction
The soldier caste in social insects, often bulkier, armoured or with greatly enlarged mandibles compared with the worker caste, defends against enemies ranging from wasps to anteaters. Eusocial thrips in the genus Kladothrips Froggatt (Order: Thysanoptera) are polymorphic insects that inhabit plant galls. The colony starts with a single foundress that rears two castes: workers, known as dispersers—because that is their ultimate goal—and soldiers. The soldier has enlarged forelimbs, thought to be for fighting a specialist invader in the genus Koptothrips Bagnall [1,2]. Studies of soldier defensive behaviour in several thrips species show that, while reluctant to attack, they will grip invaders with their forelimbs, crushing them to death [3,4]. In two species, Kladothrips intermedius Bagnall and Kladothrips habrus Mound, soldiers were significantly more effective in killing invaders than their foundresses [3]. Two subsequent studies suggested that the killing rates of soldiers of K. intermedius were similar to, or less than those of the foundress, raising doubts about the utility of soldier forelimb morphology [5,6].
We approached the defensive behaviour of soldiers from two very different directions. First, we explored the possibility that male and female soldiers behaved differently and that sex is an important variable in the outcome of combat. Secondly, we investigated the hypothesis that soldiers were as important for the defence against micro-organisms as they were against macro-organisms. While macro-organisms, such as rival thrips, wasps or even vertebrates, have been the focus of most of the literature, we concentrated on the response of soldiers to the specialist insect fungal pathogen Cordyceps bassiana.
2. Material and methods
(a). Male versus female soldiers’ fighting ability
Kladothrips intermedius soldiers and the invader, Koptothrips dyskritus, were obtained from healthy galls made by K. intermedius found on Acacia oswaldii (January 2010 and April 2011 in South Australia, 137°23′ E, 32°57′ S). An established combat procedure was used [3], in which a gall-defender and a gall-invader were placed in 200 μl PCR tubes until one combatant died. A total of 109 female soldiers and 108 male soldiers were each paired with an invader, and the proportion that survived was used to determine the overall outcome of combat. Post-battle soldiers were mounted on microscope slides and photographed using a Motic v. 2.0 digital camera and dissecting microscope. Measurements were taken using the program ImageJ (http://rsb.info.nih.gov/ij/; W. Rasband, NIH). Averages of three sets of measurements for each specimen were used for statistical analyses. Six features were measured: body length, femur length and width were taken at their maximums, pronotum length was measured along the central axis of the pronotum, width was taken at the widest posterior portion of the pronotum, and wing length from the most proximal portion of the forewing where it meets the clavus to the most distal edge of the forewing. Repeatability [7] was calculated for each morphological feature. Binary logistic regressions were executed separately for each sex to explore the possible influence of various features in predicting victories in our laboratory fighting arenas.
(b). Antimicrobial responses
Soldiers and dispersers from healthy galls of K. intermedius were washed to obtain cuticular antimicrobials using established methods [8]. As soldiers were the less numerous caste, we collected all of them from each gall and these were matched by an equal number of dispersers from that gall. Individuals of each caste were pooled from up to 10 galls to generate a sample of 100. The gender of the soldiers was not determined. This was repeated five times to yield five samples of soldiers and five of dispersers for comparison. Each was washed in 90 per cent ethanol for 5 min followed by three rinses. Ethanol was then removed by rotary evaporator and the dried extract resuspended in an exact amount of LB (Luria Bertani) broth, so the number of thrips equivalents per microlitre was known. The antimicrobial assay followed the procedures of Smith et al. [9], where a gradient of antimicrobial extract concentration was prepared across a 96-well plate starting with a concentration of 50 thrips in the first well and reducing by half in each successive well. This gradient was tested against a standard suspension of C. bassiana spores in LB. The controls for each experimental extract concentration were: (i) 100 µl extract suspension of the same concentration with 100 µl LB broth, to check for any increase in optical density (OD) owing to extract properties and (ii) wells with 100 µl of C. bassiana spore suspension added to 100 µl LB broth that provided information on normal, untreated spore germination and hyphal growth.
Plates prepared were placed in a plate reader and OD was recorded every 15 min for 48 h at 405 nm at 25°C. This provided direct comparisons between the spore germination and hyphal growth of C. bassiana treated with soldier and disperser antimicrobial concentrations with untreated spores.
To avoid issues of temporal autocorrelation, we conducted an ANOVA on OD values at two times (20 and 40 h) separately with treatment (control no. 2, dispersers, soldiers) and antimicrobial concentrations of 6.25, 12.5, 25 and 50 thrips equivalents as explanatory factors. By 20 h, most spore germination had occurred. At 40 h, the OD accurately reflected any hyphal growth. Beyond 40 h, hyphal masses led to irregular readings.
3. Results
(a). Critical body measurements
These are summarized in table 1. Male fighting forelimbs were shorter and slimmer than those of females (male length: 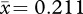 , s.d. = 0.023; female length:
, s.d. = 0.023; female length: 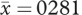 , s.d. = 0.018; t201.28 = −25.220, p < 0.001, two-tailed; male width:
, s.d. = 0.018; t201.28 = −25.220, p < 0.001, two-tailed; male width: 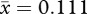 , s.d. = 0.01; female width:
, s.d. = 0.01; female width: 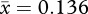 , s.d. = 0.011; t215 = −17.784, p < 0.001, two-tailed). Measurements had repeatability values near 1 (table 1), showing that variation was naturally occurring among individuals and not measurement error.
, s.d. = 0.011; t215 = −17.784, p < 0.001, two-tailed). Measurements had repeatability values near 1 (table 1), showing that variation was naturally occurring among individuals and not measurement error.
Table 1.
Summary of male and female K. intermedius soldier morphological measurements. Repeatability values of the morphological measurements were calculated using the equation R = S2A/S2W + S2A.
| measurement | sex of soldier | mean (mm) | range of values (mm) | repeatability |
|---|---|---|---|---|
| body length | female | 1.47 | 1.263–2.063 | 0.909 |
| male | 1.32 | 1.121–1.842 | ||
| wing length | female | 0.535 | 0.181–0.722 | 0.999 |
| male | 0.619 | 0.427–0.700 | ||
| femur length | female | 0.282 | 0.220–0.329 | 0.988 |
| male | 0.212 | 0.153–0.284 | ||
| femur width | female | 0.136 | 0.102–0.161 | 0.976 |
| male | 0.111 | 0.075–0.141 | ||
| pronotum length | female | 0.245 | 0.195–0.284 | 0.987 |
| male | 0.207 | 0.152–0.253 | ||
| pronotum width | female | 0.394 | 0.330–0.434 | 0.992 |
| male | 0.332 | 0.247–0.414 |
(b). Male versus female combat with invaders
No significant difference was found between male and female performance in our trials (χ2 = 2.564, d.f. = 1, p = 0.116). No morphological feature significantly altered the odds of a victory for males, but larger females with shorter wings were better at defeating invaders (body length: B = 8.149, odds ratio = 3460.8, p < 0.05; wing length: B = −5.398, odds ratio = 0.005, p < 0.05) but even in this sex, forelimb dimensions played no significant role.
(c). Antimicrobial assays
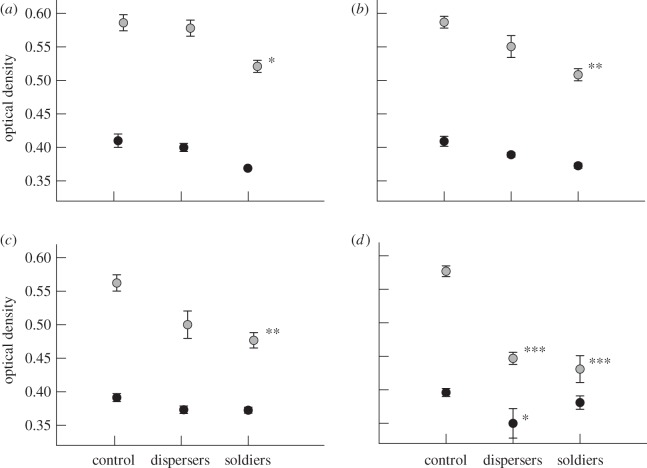
ANOVA: At 20 and 40 h, treatment was highly significant (F = 11.49, d.f. = 2, p < 0.001; F = 53.59, p < 0.001) with a strong concentration effect (F = 21.43, d.f. = 3, p < 0.001) and strong interaction between factors (F = 4.331, d.f. = 6, p < 0.001). Figure 1a,b reveals an early trend in higher antifungal activity in soldiers as opposed to dispersers, at the two lowest concentrations (Tukey's HSD = −0.057, p = 0.102). At 40 h, irrespective of concentration, there are highly significant differences between all comparisons, notably between soldiers and dispersers (Tukey's HSD =−0.0349, p = 0.001). Soldiers showed significantly more antifungal activity than dispersers, especially at low concentrations.
Figure 1.
Comparison of optical density values (mean ± s.e., n = 5) of cultures of C. bassiana after treatment with antifungal washes of disperser and soldier castes of K. intermedius. Black circles, after 20 h; grey circles, after 40 h; antifungal concentrations (a) 6.25, (b) 12.5, (c) 25, (d) 50 thrips-equivalents (control no. 2, dispersers, soldiers). Significant differences from control (Tukey's HSD): *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Despite the increased body and forelimb size of the females, they had no fighting advantage when compared with males; and our analysis within a sex found that no forelimb dimension (that we measured) was linked to success in combat. These outcomes further eroded the idea that forelimb morphology is linked strongly to the soldiers’ role in gall defence. While our experiments could not address previous findings that soldiers showed little proclivity to engage the enemy [4], this behaviour, in the context of our data, suggested that, although soldiers clearly repel other insect invaders, their apparent reluctance to engage one kind of enemy may be because resources are allocated to fight another: micro-organisms. This is supported by the antimicrobial assays, which show that soldiers strongly suppress fungal growth and, significantly, can do so at much lower densities than dispersers. This may reflect colony dynamics as soldiers are the first caste produced by the foundress and subsequently dispersers greatly outnumber them [2]. Thus, soldier antifungals may be especially critical in the early stages of colony development, where group size is low, as they may provide stronger defences than dispersers against fungal invasion. At larger group sizes, the more numerous, but weaker, dispersers may provide adequate defence.
Our results suggest that soldiers have an additional defensive role fighting micro-organisms, especially fungal pathogens, but we do not know how general this is. Cruse [10] suggested an antimicrobial role for the defensive secretions of two termite species, and this has been strongly reinforced by further experiments [11,12]. There have been many studies of social insect antimicrobial defence mechanisms [13,14], although there is currently little known about specific gall-inhabiting fungi. We have isolated one fungal pathogen from the galls of Kladothrips arotrum Mound, Kladothrips antennatus Moulton and K. intermedius (C. Turnbull 2011, unpublished data) and pathogenic fungi have been invoked for the biological control of pest thrips [14]. The strength of antimicrobials may be correlated with increasing degree of sociality and group size [8,15] and social insects have been shown to host many kinds of micro-organisms—both harmful and beneficial [16]—suggesting prolonged coevolution [17]. Entomopathogens such as Cordyceps were already present when sociality first evolved [18]. In this context, knowing that the soldiers of one thrips and several termite species secrete effective antimicrobials [10–12], it may be hypothesized that micro-organisms have been important selective agents in the evolution of the soldier caste in some social insects.
Acknowledgements
Support came from a CGS-M NSERC scholarship and a MSFSS awarded to H.E.C., an NSERC grant awarded to T.W.C., and a grant to A.J.B. by the ARC. We thank S.H., J.C., K.O. and M.O. for their assistance.
References
- 1.Crespi B. J. 1992. Eusociality in Australian gall thrips. Nature 359, 724–726 10.1038/359724a0 (doi:10.1038/359724a0) [DOI] [Google Scholar]
- 2.Crespi B. J., Morris D. C., Mound L. A. 2004. Ecology and evolution of Australian acacia thrips. In Evolution of ecological and behavioural diversity: Australian acacia thrips as model organisms (eds Korb J., Heinze J.), pp. 11–89 Canberra, Australia: CSIRO [Google Scholar]
- 3.Perry S. P., Chapman T. W., Crespi B. J., Schwarz M. P. 2004. Proclivity and effectiveness in gall defence by soldiers in five species of gall-inducing thrips: benefits of morphological caste dimorphism in two species (Kladothrips intermedius and K. habrus). Behav. Ecol. Sociobiol. 56, 602–610 10.1007/s00265-004-0811-8 (doi:10.1007/s00265-004-0811-8) [DOI] [Google Scholar]
- 4.Perry S. P., McLeish M. J., Schwarz M. P., Boyette A. H., Zammit J., Chapman T. W. 2003. Variation in propensity to defend by reproductive gall morphs in two species of gall-forming thrips. Insectes Soc. 50, 54–58 10.1007/s000400300008 (doi:10.1007/s000400300008) [DOI] [Google Scholar]
- 5.Fry S. E. 2010. Fight and flee: caste decisions during an invasion. Master's Thesis, Memorial University of Newfoundland, Canada [Google Scholar]
- 6.Caravan H. E. C. 2008. An investigation into coordinated defence in a eusocial insect colony. Honours Dissertation, Memorial University of Newfoundland, Canada [Google Scholar]
- 7.Arnqvist G., Mårtensson T. 1998. Measurement error in geometric morphometrics: empirical strategies to assess and reduce its impact on measures of shape. Acta. Zool. 44, 73–96 [Google Scholar]
- 8.Turnbull C. L., et al. 2011. Antimicrobial strength increases with group size: implications for social evolution. Biol. Lett. 7, 249–252 10.1098/rsbl.2010.0719 (doi:10.1098/rsbl.2010.0719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith S. M., Beattie A. J., Gillings M. R., Holley M. P., Stow A., Wilson P., Turnbull C. L., Briscoe D. A. 2009. An enhanced miniaturized assay for antimicrobial prospecting. J. Microb. Meth. 72, 103–106 10.1016/j.mimet.2007.10.003 (doi:10.1016/j.mimet.2007.10.003) [DOI] [PubMed] [Google Scholar]
- 10.Cruse A. 1998. Termite defences against microbial pathogens. PhD Thesis, Macquarie University, Sydney [Google Scholar]
- 11.Rosengaus R. B., Lefebvre M. L., Traniello J. F. A. 2000. Inhibition of fungal spore germination by Nasutitermes: evidence for a possible antiseptic role of soldier defensive secretions. J. Chem. Ecol. 26, 21–39 10.1023/A:1005481209579 (doi:10.1023/A:1005481209579) [DOI] [Google Scholar]
- 12.Fuller C. A. 2007. Fungistatic activity of freshly killed termite, Nasutitermes acajutlae, soldiers in the Caribbean. J. Insect. Sci. 7, 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bot A. N. M., Ortius-Lechner D., Finster K., Maile R., Boomsma J. J. 2002. Variable sensistivity of fungi and bacteria to compounds by the metapleural glands of leaf-cutting ants. Insectes Soc. 49, 363–370 10.1007/PL00012660 (doi:10.1007/PL00012660) [DOI] [Google Scholar]
- 14.Shah P. A., Pell J. K. 2003. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 61, 413–423 [DOI] [PubMed] [Google Scholar]
- 15.Stow A., Briscoe D. A., Gillings M., Holley M., Smith S., Leys R., Silberbaur L., Turnbull C., Beattie A. 2007. Antimicrobial defences increase with sociality in bees. Biol. Lett. 3, 422–424 10.1098/rsbl.2007.0178 (doi:10.1098/rsbl.2007.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie C. R., Wong B., Stuart A. E., Schultz T. R., Rehner S. A., Mueller U. G., Sung G. H., Spatafora J. W., Straus N. E. 2003. Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science 299, 386–388 10.1126/science.1078155 (doi:10.1126/science.1078155) [DOI] [PubMed] [Google Scholar]
- 17.Hughes D., Wappler T., Labandeira C. 2011. Ancient death-grip leaf scars reveal ant-fungal parasitism. Biol. Lett. 7, 67–70 10.1098/rsbl.2010.0521 (doi:10.1098/rsbl.2010.0521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung G., Poinar G., Spatafora J. 2008. The oldest fossil evidence of animal parasitism by fungi supports a Cretaceous diversification of Fungal-arthropod symbioses. Mol. Phylogenet. Evol. 49, 495–502 10.1016/j.ympev.2008.08.028 (doi:10.1016/j.ympev.2008.08.028) [DOI] [PubMed] [Google Scholar]