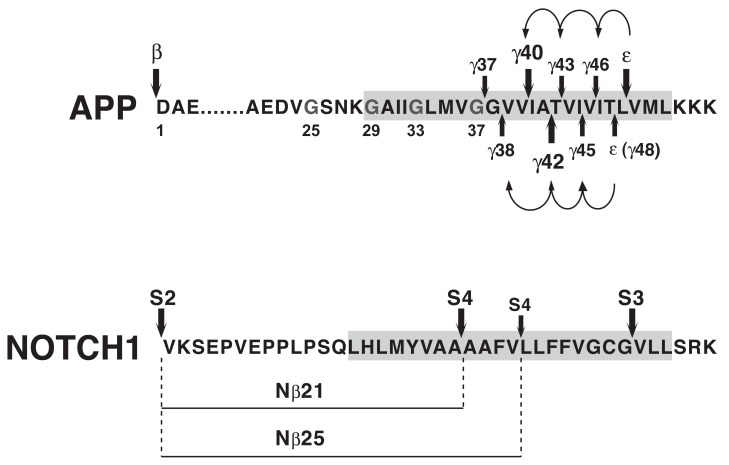
Fig. (3).
γ-secretase cleavage sites within the TMDs of APP and the NOTCH1 receptor. Cleavage by β-secretase generates the N-terminus of the Aβ sequence. Subsequently, γ-secretase cleaves within the APP TMD at multiple sites. According to the sequential cleavage model, cleavage occurs initially close to the cytosolic border of the TMD at the ε-site or at the alternative ε-site (γ48), and then in two product lines along opposite surfaces of the helical axis of the substrate APP. Most recently, direct evidence for this model has been provided with the detection of corresponding tripeptides that are released after each sequential cleavage step [68]. This study also described detection of a tetrapeptide that would explain conversion of Aβ42 to Aβ38. Evidence for conversion of Aβ40 to Aβ37 is lacking. Cleavage of NOTCH1 by ADAM10 (S2 cleavage) generates a membrane-bound, C-terminal fragment that is a direct substrate for γ-secretase. Cleavage occurs close to the cytosolic border of the TMD and results in release of the NICD domain (S3 cleavage). In addition, γ-secretase-mediated cleavage events in the middle of the TMD generate two peptides, Nβ21 and Nβ25, with differing C-termini analogous to the Aβ peptides (S4 cleavage) [113]. A similar dual-cleavage mechanism has been proposed for other substrates of γ-secretase [114, 198]. GSMs reduce the generation of Aβ42 and of the corresponding Nβ25 peptides, but spare γ-secretase cleavage at the ε-site and the S3-site and liberation of the AICD and NICD domains. This indicates that the topology of the γ-secretase cleavage sites within the TMD is crucial for the modulation of a given γ- secretase substrate by GSMs.