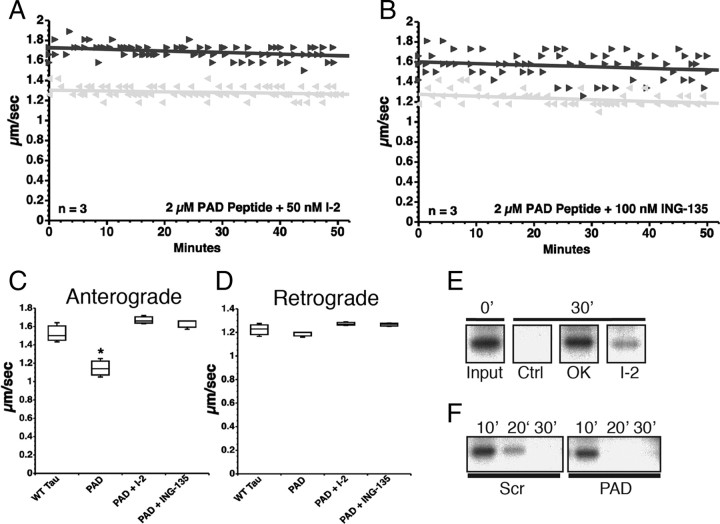
Figure 5.
PAD peptide inhibits anterograde FAT by activation of the PP1–GSK3 cascade. A, Coperfusion of PAD peptide with I-2, a specific PP1 inhibitor, had no effect on anterograde (black triangle) or retrograde (gray triangle) FAT in squid axoplasm (compare with Fig. 4B). B, Coperfusion of PAD peptide with ING-135, a specific GSK3 inhibitor, similarly prevented inhibition of anterograde FAT by PAD. C, Quantitative analysis of FAT reveals that PAD peptide alone specifically and significantly inhibits anterograde FAT (one-way ANOVA, *p > 0.01), while addition of either PP1 or GSK3 inhibitors completely prevented the reduction in anterograde FAT rates induced by the PAD peptide. D, Retrograde FAT rates were unaffected in all groups tested. E, 32P-c-Jun (a phosphatase substrate) undergoes dephosphorylation by endogenous axoplasmic phosphatases after 30 min. Axoplasms were perfused with 32P-c-Jun alone (Ctrl) or with 32P-c-Jun and either okadaic acid (OK, 1 μm) or I-2 (400 nm). Aliquots were taken immediately after perfusion (0′) and 30 min later (30′), and analyzed by autoradiography. F, 32P-c-Jun was dephosphorylated to a greater extent in PAD-perfused axoplasms than its scrambled PAD-perfused “sister” counterpart, suggesting increased activation of endogenous axoplasmic phosphatases by PAD. Sister axoplasms from the same squid were perfused with 32P-c-Jun and either scrambled PAD (Scr) or PAD peptides, and analyzed by autoradiography (n = 2 sets of sister axoplasms).