Abstract
AIM: To investigate the cellular mechanisms of action of Yiguanjian (YGJ) decoction in treatment of chronic hepatic injury.
METHODS: One group of mice was irradiated, and received enhanced green fluorescent protein (EGFP)-positive bone marrow transplants followed by 13 wk of CCl4 injection and 6 wk of oral YGJ administration. A second group of Institute for Cancer Research mice was treated with 13 wk of CCl4 injection and 6 wk of oral YGJ administration. Liver function, histological changes in the liver, and Hyp content were analyzed. The expression of α-smooth muscle actin (α-SMA), F4/80, albumin (Alb), EGFP, mitogen-activated protein kinase-2 (PKM2), Ki-67, α fetoprotein (AFP), monocyte chemotaxis protein-1 and CC chemokine receptor 2 were assayed.
RESULTS: As hepatic damage progressed, EGFP-positive marrow cells migrated into the liver and were mainly distributed along the fibrous septa. They showed a conspicuous coexpression of EGFP with α-SMA and F4/80 but no coexpression with Alb. Moreover, the expression of PKM2, AFP and Ki-67 was enhanced dynamically and steadily over the course of liver injury. YGJ abrogated the increases in the number of bone marrow-derived fibrogenic cells in the liver, inhibited expression of both progenitor and mature hepatocyte markers, and reduced fibrogenesis.
CONCLUSION: YGJ decoction improves liver fibrosis by inhibiting the migration of bone marrow cells into the liver as well as inhibiting their differentiation and suppressing the proliferation of both progenitors and hepatocytes in the injured liver.
Keywords: Yiguanjian decoction, Bone marrow transplantation, Hepatic progenitors, Hepatocytes, Hepatic injury
INTRODUCTION
Yiguanjian (YGJ) decoction, containing 6 herbs: radices glehniae, radices ophiopogonis, radix angelicae sinensis, dried rehamnnia root, lycium barbarum L and fructus meliae toosendan, was first described in an ancient book which was printed in about the 18th century. The decoction has been used clinically for almost 3 centuries for the treatment of liver diseases in China. It is reported that the decoction is effective for various hepatic diseases of different etiologies[1-3]. We previously reported that YGJ exerts significant therapeutic effects on CCl4-induced chronic liver injury in rats, through mechanisms which inhibit hepatocyte apoptosis and activation of hepatic stellate cells, as well as regulating the function of Kupffer cells[4]. Modified YGJ could also induce apoptosis in hepatic stellate cells, preventing liver fibrosis[5]. However, little is known about the molecular and cellular mechanisms of YGJ which are responsible for chronic hepatic injury. This has also limited the use of YGJ in the clinic. In this study, we aimed to investigate the cellular and molecular mechanisms of action of this decoction in suppression of hepatic fibrosis, using a CCl4-induced enhanced green fluorescent protein (EGFP) transgenic mouse model of chronic liver injury.
Liver fibrosis is a repair reaction to chronic liver injuries of varying etiologies. Many investigations have shown that bone marrow (BM) plays an important role in the progression of liver fibrosis. However it remains controversial whether the BM contributes to the promotion[6] or regression of liver fibrosis[7]. The cells in the liver derived from BM are also varied, and both parenchymal[8] and nonparenchymal cells[9-12] have been reported.
Upon liver injury, activated hepatic stellate cells (HSCs) expressing their marker protein α-smooth muscle actin (α-SMA) play a key role in the progression of hepatic fibrosis. However, the origins of HSCs are still obscure. In many studies, BM cells have been confirmed to differentiate into myofibroblasts both in vitro and in vivo. Besides liver, BM cells can contribute to the procession of many organs such as the lung, skin and kidney by virtue of differentiating into myofibroblasts in response to injury[13]. Hepatocytes are the most abundant cells in liver and are always the initial target attacked by any injury factor which contributes to their loss in number. Some reports have shown that BM cells can differentiate into hepatocytes[14] while others have shown that bone marrow-derived mesenchymal stromal cells support hepatocyte growth and proliferation[15]. Kupffer cells in liver have diverse activities, and their role in the pathogenesis of liver injury remains controversial, as it has been demonstrated that these cells display pro-toxicant and hepato-protective functions. Two major classes of Kupffer cells have been identified, but only the BM-derived class is recruited into inflammatory foci[12]. In the present study, we investigated the possibility of BM derivation of these cells in liver and the influence of YGJ on this process.
MATERIALS AND METHODS
Preparation of YGJ
All six herbs were chosen according to the Pharmacopoeia of the People’s Republic of China (2000), and authenticated by a pharmacologist. The YGJ was prepared by the Shanghai Shuguang Hospital affiliated to the Shanghai University of Traditional Chinese Medicine. Briefly, YGJ herbs, including 2000 g radices glehniae, 2000 g radices ophiopogonis, 2000 g radix angelicae sinensis, 3600 g dried rehamnnia root, 2400 g lycium barbarum L. and 900 g fructus meliae toosendan were ground into a powder, and then decocted with boiling water. The filtrate was concentrated with a rotary vacuum evaporator, and freeze-dried to 4270 g, and stored at 4 °C. Before use, the filtrate was diluted with normal saline to a final concentration of 0.2682 g/mL.
Animals
Fifty-two male 5- to 6-wk-old Institute for Cancer Research (ICR) mice and 26 EGFP+ transgenic ICR mice (license number: SCXK, 2003-0003, Shanghai, China) were purchased from the Shanghai Animal Center of the Chinese Academy of Sciences. All mice were housed in the animal center of Shanghai University of Traditional Chinese Medicine and fed a standard pelleted diet and water ad libitum.
Bone marrow transplantation plus hepatic fibrosis model
EGFP transgenic ICR 5- to 6-wk-old mice were killed by dislocation of the cervical vertebrae and used as BM donors. Approximately 0.5 × 107 whole BM cells were isolated by flushing the bones of all four limbs of EGFP donors with a gauge needle containing Roswell Park Memorial Institute 1640 medium with 2% fetal bovine serum. Normal male 5- to 6-wk-old ICR mice served as recipients, after receiving whole body irradiation of 6.2 Gy in a divided dose 2 h apart. The recipient mice received a tail vein injection of BM cells immediately after the second session of radiation.
Five weeks after transplantation, peripheral blood samples were collected and analyzed to verify successful engraftment and reconstitution of BM in transplanted mice. Twenty-four recipient mice were then injected with CCl4 as above and randomly divided into three groups: control group (n = 9), CCl4 only (n = 9) and CCl4 plus YGJ gavages for 6 wk (n = 6).
Mouse hepatic fibrosis model
Hepatic fibrosis was induced in 92 mice by subcutaneously injecting a 1:1 solution of CCl4 and olive oil (CCl4 and oil, supplied by the Chemical Agent Company of Shanghai, Shanghai, China) three times a week for 13 wk. At the end of the 7th week, CCl4-injected mice were divided into two groups: CCl4 injection only and CCl4 injection plus YGJ gavages at a dose of 0.2682 g/100 g body weight. Some mice were sacrificed at the end of the 7th, 8th, 9th, 10th, and 13th week of the experiment to dynamically observe the damage. Twelve mice served as normal controls. Blood was collected from postcaval veins for measurement of serum alanine aminotransferase (ALT) activity and albumin (Alb) content. After weighing the entire livers, liver tissue specimens were taken from the right lobe of the liver and fixed in 10% phosphate-buffered formaldehyde, then routinely processed and embedded in paraffin for histopathology, while another two samples from each mouse were embedded in optimum cutting temperature compound and snap-frozen in liquid nitrogen for immunofluorescence. The rest of the livers were stored at -80 °C.
Morphological analysis
Paraffin sections (4 μm) were hydrated and stained for 20 min in Sirius red to identify interstitial collagen. Immunohistochemical staining was performed on liver tissue sections. After deparaffinizing in xylene and dehydrating through serial alcohol solutions, we processed the sections by microwave antigen retrieval and then incubated them with monoclonal primary antibodies against α-SMA (Sigma A2547), mitogen-activated protein kinase-2 (PKM2) (Cell signaling No. 3198) and Ki-67 (Abcam ab15580) at 4 °C overnight. After washing, peroxidase-conjugated secondary antibody was added and incubated for 30 min. As a negative control, the primary antibody was replaced with phosphate buffered saline (PBS). All sections were stained with diaminobenzidine.
Immunofluorescence
After fixation in ice-cold acetone for 10 min and blocking with 10% goat serum (for Alb without serum block) for 30 min at room temperature (RT), 8 μm cryostat sections were incubated for 1.5 h at RT with a polyclonal primary antibody against green fluorescence protein (GFP) (Cell Signaling, No. 2555). After rinsing with PBS, the secondary antibody, Alexa Fluor 488 goat anti-rabbit IgG (H + L) (Invitrogen A11008) was added and sections were incubated for 1 h at 37 °C.
For detection of α-SMA, the GFP-conjugated sections were blocked with 10% goat serum and incubated with monoclonal α-SMA (sigma A2547) at a dilution of 1:200 overnight at 4 °C. The secondary antibody Alexa Fluor 633 goat anti-mouse (Invitrogen A21050) was used at a dilution of 1:200 for 1 h at 37 °C.
For detection of Alb, 8 μm cryostat sections were fixed in ice-cold acetone for 10 min, then incubated with the primary antibody, a goat polyclonal antibody (Santa Cruz Biotechnology, INC sc-46293), at 1:50 overnight at 4 °C. After rinsing, the secondary antibody, donkey anti-goat 633 (Invitrogen A21082) was added at 1:200 for 1 h at 37 °C. Following blocking with 5% BSA, the antibody to GFP and the Alexa Fluor 488-labeled secondary antibody described above were used as primary and secondary antibodies, respectively.
All the slides were washed 3 times for 5 min each in PBS between primary and secondary antibodies. The slides were mounted in a mixture of PBS and glycerol.
Microscopy and image capture
For light microscopy, an Olympus IX70 microscope was used with Image-Pro Plus 6.1 image analysis software. For fluorescent microscopy, slides were visualized under an Olympus CKX41 fluorescent microscope. For confocal microscopy, a Leica laser confocal microscope equipped with a triple bandpass filter was used. The images were analyzed with ScnImage and quantified using the control group as the baseline.
Liver function and hydroxyproline
Liver Hyp content, serum ALT activity and Alb content were determined according to the protocols provided with the kits, which were supplied by the Institute of Biological Products Nanjing Jiancheng (Nanjing, China).
RNA isolation and real-time polymerase chain reaction
Total RNA was isolated with Trizol Reagent (Invitrogen lot: 1401902) and the quality was checked by spectroscopy. The A260/A280 ratio was between 1.9 and 2.1 for all samples.
For the reverse transcription reaction, cDNA was produced using a Revert Aid™ First Strand cDNA Synthesis Kit (Ferments Life Sciences, No. K1 621). Real-time polymerase chain reaction (PCR) was performed using a real-time PCR machine (Rotor-Gene RG-3000, Corbett Research) in quantitative mode. Initial denaturation was performed at 95 °C for 10 s, followed by 40 cycles of 95 °C denaturing for 5 s, primer annealing, and extension at 62 °C for 20 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. The templates and primers were as follows: mouse monocyte chemiotaxis protein (MCP)-1 (Forward: 5’-TCAGCCAGATGCAGTTAACGC-3’ and Reverse: 5’-TCTGGACCCATTCCTTCTTGG-3, 185 bp); CC chemokine receptor 2 (CCR2) (Forward: 5’-CACGAAGTATCCAAGAGCTT-3’, and Reverse: 5’-CATGCTCTTCAGCTTTTTAC-3’, 199 bp) and they were normalized to GAPDH (Forward: 5’-GAGCGAGACCCCACTAACAT-3’ and Reverse: 5’-TCTCCATGGTGGTGAAGACA-3’, 86 bp). The SYBR Green fluorescence intensity of the specific double-strand reflecting the amount of amplicon formed was read after each elongation step at an additional acquisition temperature using Rotor-Gene Analysis Software V6.0. To verify the specificity of the amplification reaction, melting curve analysis was performed. For quantification analysis, all samples were analyzed in triplicate, and cycle threshold values for target genes and the housekeeping gene were determined for each sample. Relative gene expression was presented using the 2-∆∆CT method.
Western blotting
Liver samples were homogenized and the supernatants were collected after centrifugation at 12 000 × g at 4 °C for 10 min. Protein concentrations were determined using a protein assay kit II (BIO-RAD, No. 500-0 122), and 50 μg proteins were loaded and separated on 12% sulfate dodecyl sodium-polyacrylamide gels. The proteins were electrotransferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, United States) in transfer buffer at 4 °C for 1 h. Nonspecific binding to the membrane was blocked for 1 h at room temperature with 5% nonfat milk in Tween-80 Tris-buffered saline. The membranes were then incubated overnight at 4 °C with various primary antibodies in blocking buffer containing 5% nonfat milk, followed by incubation with horseradish peroxidase-conjugated secondary antibody (Chemicon Co) for 1 h in 5% nonfat milk dissolved in Tris-buffered saline. Membranes were then washed with Tris-buffered saline, signals were visualized using the enhanced chemiluminescence system (Amersham Biosciences) and the intensity of the bands was determined by scanning video-densitometry and normalized to the control group.
Statistical analysis
All results were expressed as mean ± SD. Data were analyzed using one-way analysis of variance. Student’s t test was employed for the comparison of parameters and correlation analysis, respectively.
RESULTS
YGJ decoction dynamically protects against carbone tetrachloride-induced chronic liver fibrosis
Changes in serum ALT activity in the mice receiving CCl4 which developed chronic liver injury were evaluated at several distinct time points. From week 7 to week 13, serum ALT activity in the CCl4-injected mice increased continually and significantly compared with that in the control group (P < 0.01), but YGJ markedly reduced serum ALT activity even after just one week of administration (P < 0.05), and ALT was reduced to 50% of that in the control CCl4-injected group (P < 0.01) after 6 wk of YGJ administration (Figure 1).
Figure 1.
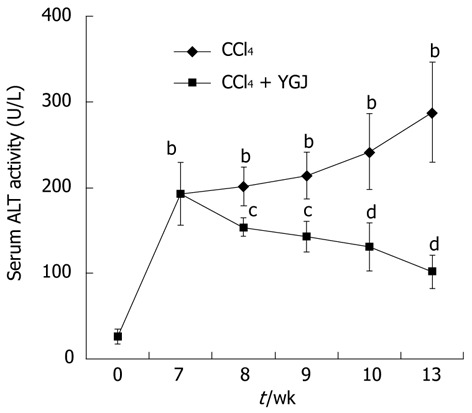
Serum alanine aminotransferase activity in mice. ALT activities in CCl4 groups increased continuously but declined in YGJ + CCl4 groups over the entire period. bP < 0.01 vs the week 0 control group; cP < 0.05, dP < 0.01 vs the same time-point CCl4 group. Results are presented as mean ± SD. ALT: Alanine aminotransferase; CCl4: Carbone tetrachloride; YGJ: Yiguanjian.
The results of Sirius red staining showed that only a little collagen was present in the area of the portal and central veins in normal mice, while over the course of the study, bridging collagen connecting the central veins and neighboring portal areas increased continually, deposition of collagen fibers was steadily enhanced in livers throughout the entire experimental period, and pseudo- nodules were formed by the end of the study. In contrast, in the YGJ-treated group, collagen deposition in the livers was significantly attenuated. Semi-quantification of the collagen staining showed that collagen levels increased sharply after CCl4 injection, reaching 3.5-fold above basal values by week 7, and peaking 6.5-fold above basal values by week 13. YGJ treatment maintained and significantly reduced the increase in collagen. For example, after 6 wk of YGJ treatment, the level of collagen was about 50% lower in YGJ-treated mice than in controls at the same time point (Figure 2A and B).
Figure 2.
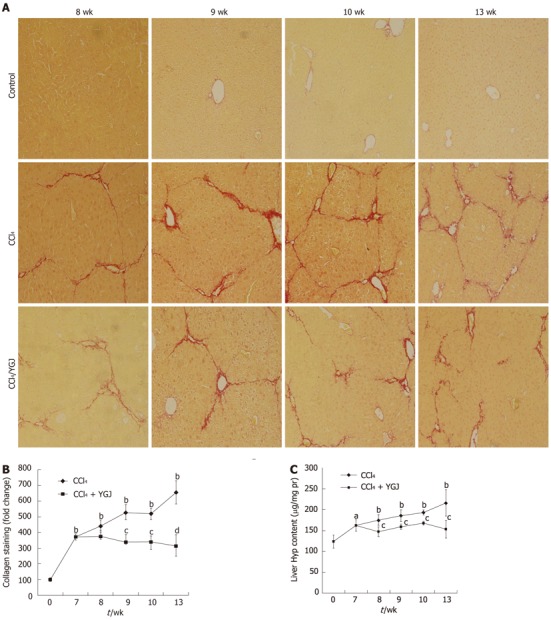
Collagen staining with Sirius red and Hyp content. A: Collagen staining. Week 0 control mice have limited collagen in the portal area, but fibrous septa bridging the portal tracts were found in the livers of CCl4-treated mice, and this was significantly attenuated in YGJ-treated mice, × 200; B: Semi-quantification of collagen staining, the week 0 control group level was set as the basal level; C: Hyp content in the liver. Results of collagen staining and Hyp content show that they both continuously increased in the CCl4-treated group but declined in the YGJ + CCl4 group over the entire period. aP < 0.05, bP < 0.01 vs week 0 control group; cP < 0.05, dP < 0.01 vs the same time-point CCl4 group. Results are presented as mean ± SD. CCl4: Carbone tetrachloride; YGJ: Yiguanjian; Hyp: Hydroxyproline.
The results of Sirius red staining also showed that Hyp concentration in livers was significantly increased from week 8 and increased steadily up to week 13. However, in the YGJ-treated group, the content of Hyp in livers significantly decreased as compared with that at the same time-point in the CCl4-injected group (Figure 2C). These findings support our previous results showing that YGJ protected hepatic function and attenuated collagen deposition during chronic liver injury in rats[4].
YGJ decoction continuously inhibits α-smooth muscle actin (+) cells in chronically-injured liver
During development of chronic liver injury, accumulation of myofibroblastic cells and increased production of extracellular matrix were the main characteristics. It is clear that α-SMA-positive activated HSCs, the main source of extracellular matrix, play a key role in this pathological process. Our studies of immunohistochemical staining showed that the accumulation in livers of the myofibroblast marker α-SMA increased steadily and then remained at this level until the end of the study, peaking at the 13th week when it was distributed along fibrotic areas (Figure 3A and B). This change was consistent with the expression of α-SMA protein analyzed by Western blotting, which also increased after CCl4 injection and peaked at the end of the study (Figure 3C and D). The progressive expression of α-SMA in this study is consistent with earlier reports which demonstrated that early increases in the number of activated HSCs, followed by enhanced collagen synthesis and decreased collagen degradation, combined to cause accumulation of collagen matrix following liver injury. YGJ treatment significantly attenuated the induction of α-SMA, even after only 1 wk of YGJ administration (P < 0.01) and it continually declined up to 6 wk of YGJ gavages (P < 0.01) (Figure 3A and B), a result which was similar to that obtained from the Western blotting of α-SMA in livers (P < 0.01) (Figure 3C and D).
Figure 3.
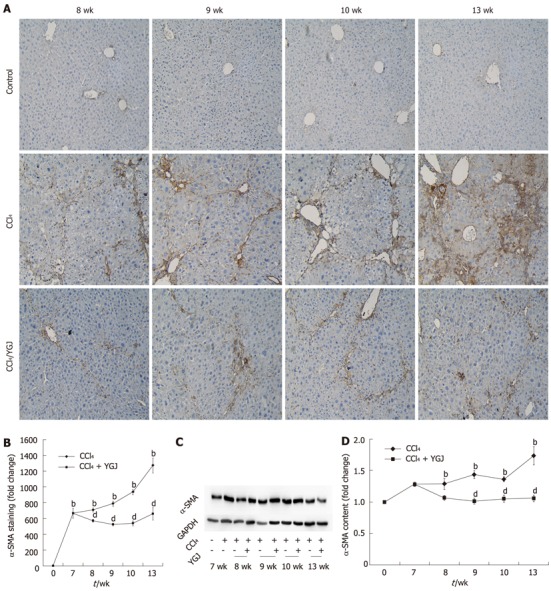
Immunostaining and Western blot analysis of α-smooth muscle actin in liver tissues. A: α-smooth muscle actin (α-SMA) immunostaining, × 200; B: Semi-quantification of α-SMA positive area, with the level in the week 0 control group set as the basal level; C: Western blotting of α-SMA; D: Semi-quantification of the α-SMA Western blot results, with the week 0 control group level set as the basal level. bP < 0.01 vs week 0 control group; dP < 0.01 vs the same time-point CCl4 group. Results are mean ± SD. CCl4: Carbone tetrachloride; YGJ: Yiguanjian; GAPDH: Glyceraldehydes-3-phosphate dehydrogenase.
YGJ decoction inhibits the migration of bone marrow cells into chronically injured liver
It has been reported that bone marrow cells or their progeny can circulate into various damaged organs and differentiate into myofibroblasts or fibrocytes[13]. In the liver, several studies have suggested that bone marrow contributes to scar forming cells of various types, such as the HSCs[10]. Our results further confirmed these reports. Following CCl4 injection into the mice which received bone marrow transplantation, the number of EGFP+ cells also increased steadily in injured livers, which were mainly distributed in the fibrotic areas. In contrast, only a few EGFP+ cells were found in the vehicle-treated control mice, who received only olive oil but no CCl4 injection after bone marrow reconstitution. In YGJ-treated mice, the number of EGFP+ cells decreased markedly as compared with the CCl4-injected mice at the same time point. Moreover, unlike the distribution of EGFP+ cells which were mainly found in the fibrotic area in CCl4-injected mice, the EGFP+ cells were distributed not only in mesenchymal but also in parenchymal areas in YGJ-treated mice (Figures 4, 5, and 6).
Figure 4.
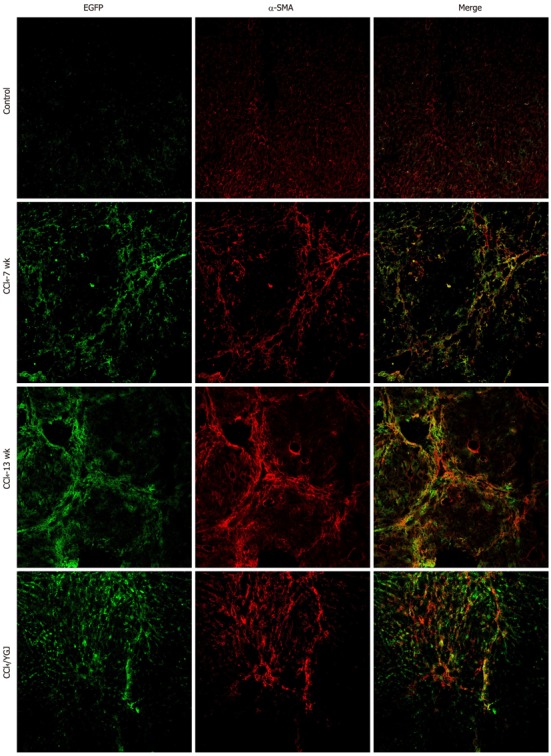
Immunofluorescent colocalization of enhanced green fluorescent protein and α-smooth muscle actin in CCl4-induced chronic liver injury in bone marrow chimera mice, × 200. EGFP is shown in green and α-smooth muscle actin (α-SMA) in red. A yellow color confirms colocalization in the merged images. The number of EGFP/α-SMA double positive cells was found to increase in all mice after receiving CCl4 injection and they were mainly found in the areas of scarring. In contrast, the number of EGFP+/α-SMA+ cells decreased in the YGJ treatment group. EGFP: Enhanced green fluorescent protein; YGJ: Yiguanjian.
Figure 5.
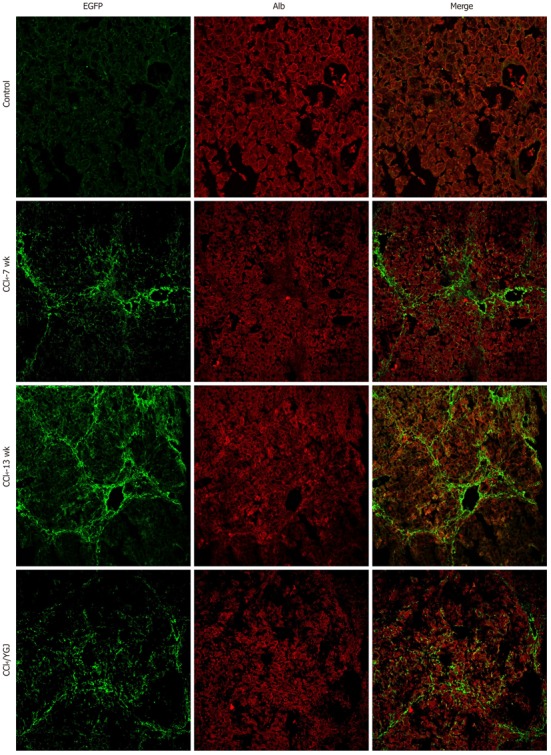
Immunofluorescent colocalization of enhanced green fluorescent protein and albumin in CCl4-induced chronic liver injured bone marrow chimera mice, × 200. EGFP is shown in green and Alb in red. No yellow color is visible in the merged images in control, CCl4 or CCl4 + YGJ groups. EGFP: Enhanced green fluorescent protein; YGJ: Yiguanjian; Alb: Albumin.
Figure 6.
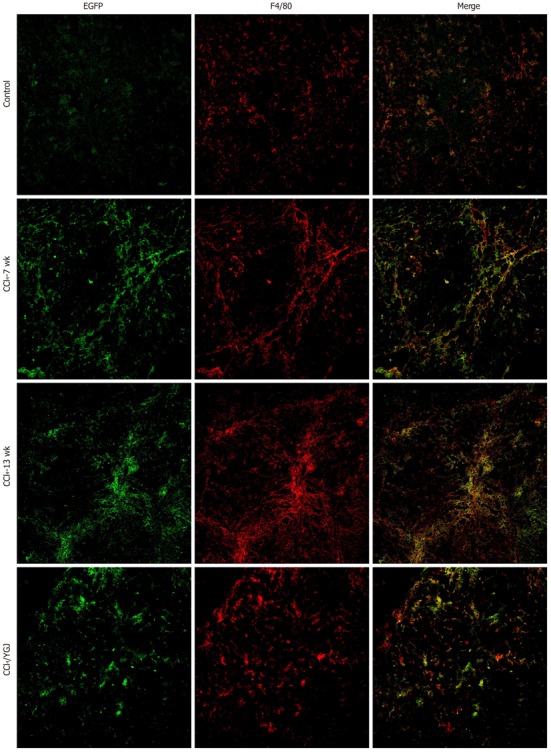
Immunofluorescent colocalization of enhanced green fluorescent protein and F4/80 in CCl4-induced chronic liver injured bone marrow chimera mice, × 200. EGFP is shown in green and F4/80 in red. A yellow color confirms colocalization in the merged images. The number of EGFP/F4/80 double positive cells was found to increase in all mice after receiving CCl4 injection. In contrast, the number of EGFP+/F4/80+ cells decreased in the YGJ treatment group. EGFP: Enhanced green fluorescent protein; YGJ: Yiguanjian.
YGJ decoction-mediated bone marrow-derived cell differentiation in chronically injured liver
In accordance with the results of the dynamic CCl4 injection model without BM transplantation, both the number and distribution of α-SMA+ cells increased constantly over the course of liver injury in mice with bone marrow reconstitution. Treatment with YGJ reduced the number of α-SMA+ cells in fibrotic livers. The results of EGFP/α-SMA double staining showed that there were almost no double positive cells in the vehicle control mice. In contrast, the number of the EGFP+/α-SMA+ cells increased over time in all mice after receiving CCl4 injections for 7 wk (P < 0.01) and remained high to the final time-point (P < 0.01); moreover, the EGFP+/α-SMA+ cells were mainly found in the areas of scarring. However, YGJ treatment significantly decreased the number of EGFP+/α-SMA+ cells as compared with the CCl4-injected controls at the same time-points (P < 0.01) (Figure 4).
Because of the apparent increase in the number of EGFP+ cells in parenchymal areas in YGJ-treated mice compared with CCl4-injected mice, we hypothesized that YGJ could stimulate the EGFP+ cells from bone marrow to differentiate into hepatocytes. To assess the possibility of YGJ stimulating bone marrow cells to differentiate into hepatocytes, we investigated the presence of EGFP+/Alb+ cells by double staining. In the CCl4-injected control mice, the number of EGFP+ cells increased steadily over time and the cells were distributed mainly in mesenchymal areas, but Alb+ cells were mainly distributed in parenchymal areas, and there was no overlap between EGFP+ cells and Alb+ cells, indicating that no EGFP+/Alb+ cells were present in chronically injured liver. No EGFP+/Alb+ cells were present in the vehicle control mice or in the YGJ-treated mice (Figure 5). These results seemingly suggest that no hepatocytes were derived from bone marrow in our study.
To further investigate the mechanisms of YGJ mediation of bone marrow cell differentiation in chronic liver injury, we explored the possibility that BM cells differentiated into other main types of cells in the liver, such as Kupffer cells. The EGFP+/F4/80+ cells represented BM-derived Kupffer cells in our experiments. In the vehicle control mice, despite of a little F4/80 expression, virtually no EGFP+/F4/80+ cells were present in the liver. However, after 7 wk of CCl4 injection, coexpression of EGFP and F4/80 increased significantly (P < 0.01) and remained at a high level up to 13 wk. These results indicated that some Kupffer cells differentiated from BM cells as the hepatic injury progressed. With YGJ treatment, both EGFP and F4/80 were expressed in parenchymal areas, and some of the areas were overlapped (Figure 6). However, there were fewer EGFP+/F4/80+ cells in the YGJ-treated mice than in the CCl4-injected mice (P < 0.01).
YGJ decoction suppresses monocyte chemotaxis protein-1 and CCR2 expression in fibrotic liver
MCP-1 was identified as a factor likely to be responsible for stem/progenitor cell recruitment. Therefore, we analyzed the expression of MCP-1 during CCl4-induced fibrogenesis. For the gene and protein expression, results were normalized to expression in the liver at the time of CCl4 injection (0 wk), for example the control group mRNA level of MCP-1 reached 2.8-fold after 7 wk (P < 0.05) and 6.7-fold after 13 wk of CCl4 injection (P < 0.05) (Figure 7A). Western blotting showed that MCP-1 protein was increased markedly (approximately 4 fold) after 13 wk of CCl4 injection (Figure 7A and B). YGJ treatment significantly reduced the expression of both MCP-1 gene and protein (Figure 7A and B). CCR2 is the specific receptor of MCP-1, and both Kupffer cells and HSCs, but not hepatocytes, have been shown to express CCR2 in liver[16]. In our study, the gene expression of CCR2 continuously increased over the course of the experiment (P < 0.05), which was in accordance with its protein expression (P < 0.01), and treatment with YGJ only suppressed expression at the protein level (P < 0.05) but not at the gene level (Figure 7B and C). These data demonstrate that more exogenous stem/progenitor cells were recruited into chronically injured livers compared with the controls, but that YGJ treatment decreased the number of exogenous stem/progenitor cells in the liver.
Figure 7.
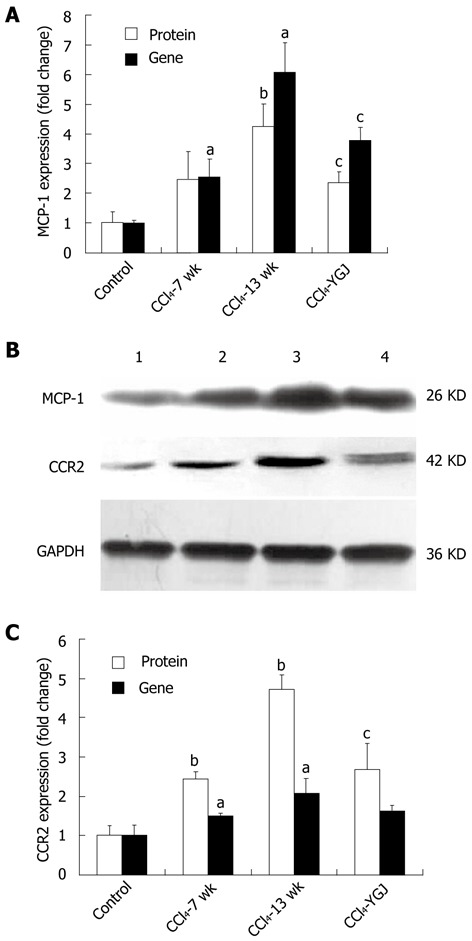
Gene expression analyzed by real-time polymerase chain reaction and protein expression analyzed by Western blotting of monocyte chemotaxis protein-1 and CC chemokine receptor 2 in CCl4-induced chronic liver injured bone marrow chimera mice. A: MCP-1 gene and protein expression. For both genes the relative expression is quantified using the control group as the basal level; B: MCP-1 and CCR2 bands by Western blotting. Band 1, control group; band 2, CCl4 7 wk group; band 3, CCl4 13 wk group; and band 4, CCl4 + Yiguanjian (YGJ) group; C: CCR2 gene and protein expression. For both genes the relative expression is quantified using the control group as the basal level. MCP-1: Monocyte chemotaxis protein-1; CCR2: CC chemokine receptor 2; GAPDH: Glyceraldehydes-3- phosphate dehydrogenase. aP < 0.05, bP < 0.01 vs week 0 control group; cP < 0.05 vs the same time-point CCl4 group.
YGJ decoction decreases proliferation of liver epithelial progenitor cells and mature hepatocytes
It is important to determine whether the proliferative activity of hepatocytes increased during hepatic injury since the hepatocytes are the most numerous cells in liver and have potent proliferative activity. Ki-67 nuclear accumulation increased constantly in hepatocytes after liver injury, reaching 23-fold as compared with the basal level of controls at week 13, the end point of the study (Figure 8A and B). This finding means that the number of mature hepatocytes increased steadily and significantly after CCl4 injury, and indicates that mature hepatocytes also play a part in responding to CCl4-induced liver injury by increasing their proliferative activity. However, even after 1 wk of YGJ treatment, the number of Ki-67 positive hepatocytes was dramatically reduced (P < 0.01), and by the 13th wk, the number accounted for only 12.6% of those in the CCl4 injection group at the same time point, which is a reduction of approximately 87% compared with the CCl4-treated group (Figure 8A and B). These findings suggest that YGJ treatment significantly reduced the number of hepatocytes accumulated after CCl4 injury.
Figure 8.
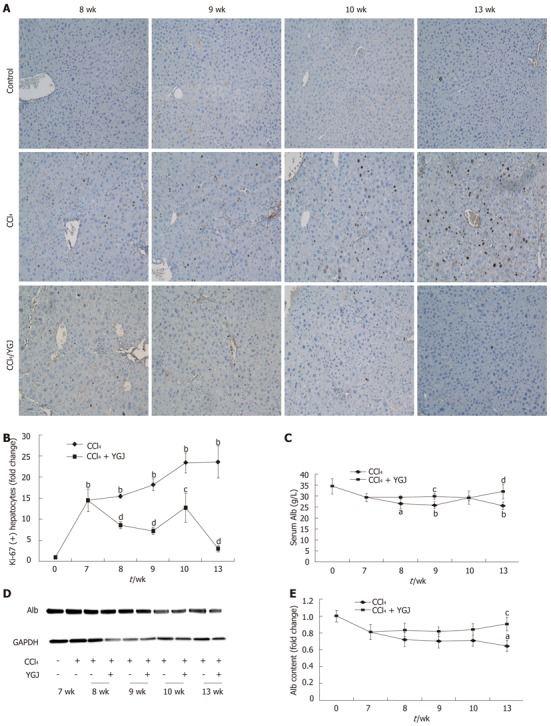
Hepatocyte function. A: Ki-67 immunostaining in liver tissues, × 200; B: Semi-quantification of Ki-67 staining, with the week 0 control group level as the basal level; C: Serum Alb content; D: Liver Alb Western blotting bands; E: Semi-quantification of Alb based on Western blotting results, with the week 0 control group level as the basal level. aP < 0.05, bP < 0.01 vs week 0 control group; cP < 0.05, dP < 0.01 vs the same time-point CCl4 group. Results are presented as mean ± SD. GAPDH: Glyceraldehydes-3-phosphate dehydrogenase; YGJ: Yiguanjian; Alb: Albumin.
Serum Alb concentration (34.5 ± 6.9 g/L, control group) decreased by week 7 after hepatic injury (29.4 ± 3.5 g/L), and continued to decrease steadily at week 8 (26.4 ± 4.5 g/L, P < 0.05), week 9 (25.3 ± 3.2 g/L, P < 0.01), week 10 (28.9 ± 2.2 g/L) and week 13 (25.5 ± 2.40 g/L, P < 0.01). However, in YGJ-treated mice, the serum Alb concentration increased markedly compared with that at week 9 (P < 0.05) and week 13 (P < 0.01) (Figure 8). Similarly, Western blotting results of Alb also showed that Alb protein expression in injured livers continually declined and reached its lowest level by the end of the experiment, at only 64% of the basal level. In contrast, treatment with YGJ steadily enhanced its protein level so that it reached 90% of the basal level by the end of the experiment.
During the process of liver regeneration after injury, liver epithelial progenitor cells were induced to proliferate to compensate for the missing number of parenchymal hepatocytes. Hence, hepatic expression of progenitor markers, such as AFP and PKM2[17,18], were used in our experiment to assess the proliferation of hepatic progenitor cells after liver injury. PKM2 expression increased steadily after CCl4 injection as shown by immunostaining and Western blotting analysis, spanning 6 time points over 13 wk. Our data revealed that after 7 wk of CCl4 injection, PKM2 expression increased significantly (P < 0.01) and thereafter remained at a high level throughout the entire experimental period (Figure 9A, B and D). These enhancements were accompanied by an increase in AFP expression (Figure 9C and E). Treatment with YGJ significantly attenuated the increase in the progenitor markers PKM2 and AFP (Figure 9). These findings support the concept that after CCl4 injection, hepatic injury promotes accumulation of liver progenitor cells.
Figure 9.
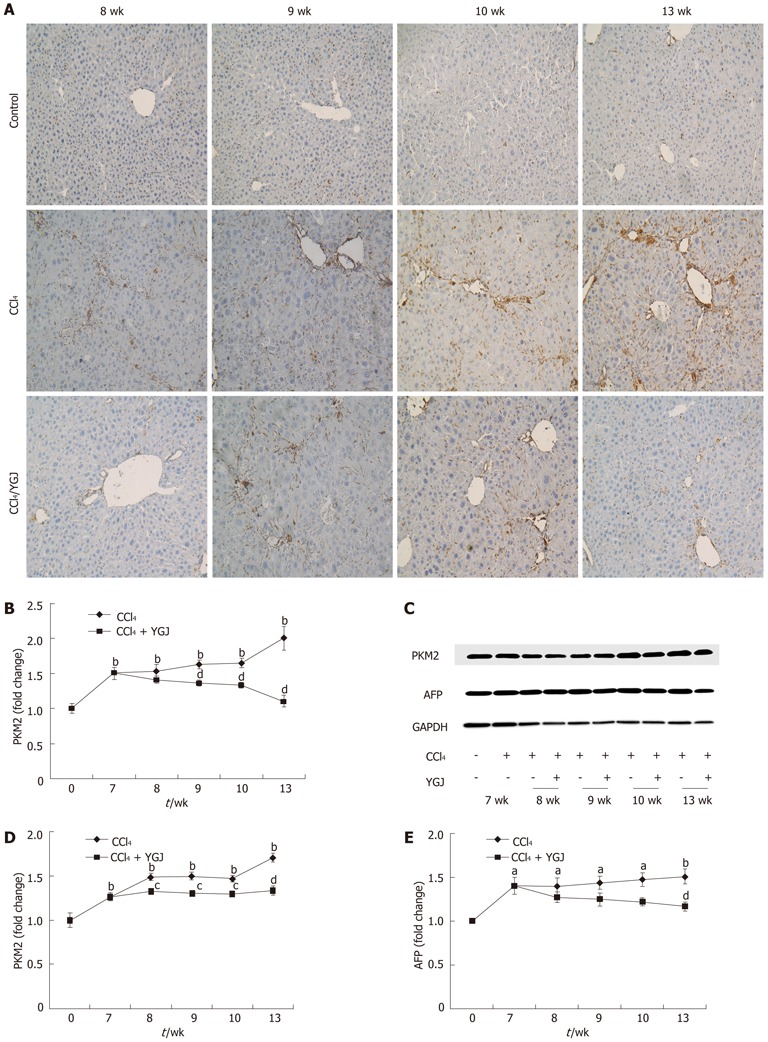
Expression of progenitor markers in liver tissues. A: PKM2 immunostaining, × 200; B: Semi-quantification of PKM2 staining, with the week 0 control group level as the basal level; C: PKM2 and AFP Western blotting bands; D: Semi-quantification of PKM2 in Western blotting results, with the week 0 control group level as the basal level; E: Semi-quantification of Western blotting results of PKM2, with the week 0 control group level as the basal level. aP < 0.05, bP < 0.01 vs week 0 control group; cP < 0.05, dP < 0.01 vs the same time-point CCl4 group. Results are presented as mean ± SD. GAPDH: Glyceraldehydes-3-phosphate dehydrogenase; YGJ: Yiguanjian; PKM2: Mitogen-activated protein kinase-2; AFP: α fetoprotein.
DISCUSSION
YGJ can ameliorate chronic liver injury in some animal models of CCl4-induced liver damage in mice. In the course of the experiment, serum ALT activity, deposition of collagen fibers and Hyp content were continuously increased, and had formed pseudo-nodules by the end of the study. In contrast, all these markers decreased significantly after YGJ treatment. These results strongly suggest that YGJ can block CCl4-induced chronic liver injury and exhibit a favorable therapeutic effect in mice.
Any etiologically chronic liver injury could result in activation of myofibroblasts, which are the main source of extracellular matix (ECM) and finally lead to fibrosis or cirrhosis. We consider myofibroblasts to be a target of therapeutic liver fibrosis because they play such a key role in liver fibrogenesis. In our mouse model, α-SMA expression in mice increased in a dynamic manner after CCl4 injection, and reached a peak at week 13. In contrast, α-SMA expression was remarkably and constantly suppressed after YGJ treatment.
Because myofibroblasts are thought to be heterogeneous in origin, both intrahepatic and BM-derived sources are important in the development of fibrosis[19]. To assess the function of the BM in supplying myofibroblasts in cases of chronic liver injury, we examined the formation of BM-derived myofibroblasts and found that BM-derived EGFP-positive cells time-dependently increased over the course of the study, reaching a peak at week 13, and they were mainly localized along the fibrous septa, in accordance with the course of fibrogenesis. Furthermore, the number of EGFP and α-SMA double positive cells increased time-dependently and they were scattered at the fibrous septa. Therefore, we concluded that the EGFP and α-SMA positive cells are of BM origin and that BM cells can migrate and differentiate into myofibroblasts in the damaged liver. On the other hand, the number of both EGFP-positive cells and the EGFP-α-SMA positive cells decreased steadily after YGJ treatment, which suggested that YGJ inhibited the migration and differentiation of BM cells into myofibroblasts.
Kupffer cells facilitate liver fibrogenic processes either by secreting fibrotic factors or by increasing the production of tissue inhibitor of metalloproteinases to reduce ECM degradation[20]. The origin of Kupffer cells was thought to be recruitment from the bone marrow to the liver[12] or from intrahepatic precursor cells that exist in the liver[21]. However, only the former population can be recruited into inflammatory foci in response to inflammation[22]. Our results indicate that the number of EGFP and F4/80 double positive cells begins to increase after CCl4 injection, reaching a peak at week 13, and that the cells are distributed along the fibrotic septa. The number of EGFP and F4/80 double positive cells decreased significantly after YGJ treatment. This revealed that YGJ inhibited liver fibrogenesis by mediating BM differentiation into Kupffer cells in the liver.
It has also been reported that various components of the bone marrow can differentiate into hepatocyte-like cells, causing a decrease in liver fibrosis[23,24]. The results from previously published reports concerning whether hepatocytes were BM-derived or not were conflicting. Our results revealed virtually no EGFP-Alb double positive cells in any of the mice, whether they were in the control, CCl4-damaged or YGJ treatment group.
Chemokines and their receptors play a central role in the regulation of cell migration. MCP-1 and its receptor CCR2 not only contribute to the recruitment of BM cells to liver but also have been identified as profibrotic mediators[25,26]. Increased MCP-1 is associated with HSCs and macrophage recruitment[27,28]. In the present study, MCP-1 increased constantly over the course of fibrogensis. Bone marrow F4/80+ cells are the major producers of MCP-1[29], and we observed that the number of bone marrow F4/80+ cells increased following liver injury. These results revealed that production of MCP-1 by bone marrow F4/80+ cells recruited into liver was one of the mechanisms underlying CCl4-induced liver fibrosis. YGJ treatment significantly decreased the number of bone marrow F4/80+ cells and the level of MCP-1 expression in accordance with the improvement of liver fibrosis.
CCR2 promotes fibrosis[30,31] and is expressed on resident liver cells including hepatic stellate cells and Kupffer cells, but not hepatocytes[16]. CCR2 expression increased over the time-course of CCl4 injection, similarly to MCP-1 expression. YGJ treatment markedly inhibited CCR2 expression.
In order to further investigate the mechanisms of YGJ antifibrotic function in CCl4 injury, we took into account the role of mature hepatocytes. Because various etiologies of liver injury involve hepatocytes as the target, mature hepatocytes have stem cell functions, giving them the ability to re-enter the cell cycle rapidly after liver injury has occurred[32]. The number of proliferative hepatocytes increased constantly after CCl4 injection, reaching a peak at week 13. Unlike the proliferative behavior of mature hepatocytes during injury, both the serum content and expression of Alb protein, which is uniquely synthesized by hepatocytes, in the liver declined continuously over the time course of liver injury. It would be reasonable to assume that the proliferation of hepatocytes in injured liver is a kind of compensatory response to their reduction in number. In contrast, hepatocyte proliferation was suppressed steadily after YGJ administration, but the Alb content in both serum and liver increased continuously. It seems unnecessary to initiate mature hepatocyte proliferation after YGJ treatment, since YGJ works as an inhibitor of hepatocyte apoptosis in CCl4-induced liver fibrosis[4]. Together with the ALT results, these findings infer that the improvement in hepatocyte function in the YGJ group may result from attenuation of hepatocyte necrosis or apoptosis.
Hepatic progenitor cells (or oval cells) are activated in response to persistent liver injury when the hepatocytes are unable to mount a proliferative response to injury[32]. In the present study, AFP and PKM2 protein expression increased continuously with time after CCl4 injection, reaching a peak at the 13th week of CCl4 injury. The results demonstrated that hepatic progenitor cells were activated in the CCl4-injured liver, but reduced steadily after oral YGJ administration. These results suggest that hepatic progenitor cells are activated by persistent CCl4-induced liver injury, but the hepatic progenitor cells are not activated after YGJ treatment.
In summary, our results revealed that YGJ improves CCl4-induced liver injury by inhibiting BM migration into injured liver and suppressing the proliferation of hepatic progenitor cells and hepatocytes.
COMMENTS
Background
Liver fibrosis is usually progressive and reversible, but up to now, the molecular and cellular mechanisms responsible for the reversibility of liver fibrosis have been poorly understood. Yiguanjian (YGJ) decoction has been employed clinically in China for centuries to treat various chronic hepatic injuries, but its mechanisms of action remain unclear.
Research frontiers
YGJ decoction is a traditional Chinese medicine complex containing six herbs which has a long history of clinical use. In the search for therapies for chronic liver diseases, the research hotspot focuses on its mechanisms of eliminating liver fibrosis.
Innovations and breakthroughs
In previous published papers, research focuses on how YGJ improves liver fibrosis, and especially how it induces apoptosis of hepatic stellate cells while exerting anti-apoptotic effects in hepatocytes. In the present study, the authors not only investigated the homing of bone marrow cells to the liver and their differentiation, but also investigated the behavior of intrahepatic progenitors and mature hepatocytes during liver injury. The number of bone marrow cells which differentiate into fibrogenic cells increased in the liver following injury, but was decreased by YGJ treatment. Furthermore, the proliferation of intrahepatic progenitors and mature hepatocytes was stimulated during CCl4-induced liver injury, and YGJ also suppressed their proliferation.
Applications
The study results suggest that oral administration of YGJ decoction is a potential therapy for the prevention of chronic liver injury of varying etiologies.
Terminology
Liver fibrosis is a pathological progress characterized by an excessive deposition of extracellular matrix especially collagen; YGJ is a traditional Chinese medicine containing six herbs, which has a long history of clinical use in China.
Peer review
This is a good descriptive study in which authors analyze the effects of Yiguanjian decoction in liver fibrosis, induced by CCl4. The results are interesting and suggest that YGJ decoction improved liver fibrosis by inhibiting the migration of bone marrow cells into the liver as well as inhibiting their differentiation and suppressing the proliferation of both progenitors and hepatocytes in the injured liver. The paper is interesting and deserves to be published.
Footnotes
Supported by National Natural Science Foundation of China, No. 30772758; and National Science and Technology Major Project of China, No. 2009ZX09311-003
Peer reviewer: Dr. Ricardo Marcos, Laboratory Histology and Embryology, Institute of Biomedical Sciences Abel Salazar, Lg Prof Abel Salazar, 4099-003 Porto, Portugal
S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
References
- 1.Xiong XJ, Li HX. Experience on clinical application of Chinese herbal medicine Yi Guan Jian decoction. Zhongxiyi Jiehe Xuebao. 2011;9:920–923. doi: 10.3736/jcim20110815. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Liu JL, Tian FF. Treating 50 cases of hepato-cirrhosis from hepatitis B with Yin Chen Hao soup plus Yi Guan Jian. Zhonghua Shiyong Zhongxiyi Zazhi. 2007;8:689. [Google Scholar]
- 3.Yan ZL, Lin H. Clinic study of YGJ decoction combind with adefovirdipivoxil treatment chronic hepato-fibrosis from hepatitis B. Zhongguo Minzu Minjian Yiyao Zazhi. 2010;19:115–116. [Google Scholar]
- 4.Mu Y, Liu P, Du G, Du J, Wang G, Long A, Wang L, Li F. Action mechanism of Yi Guan Jian Decoction on CCl4 induced cirrhosis in rats. J Ethnopharmacol. 2009;121:35–42. doi: 10.1016/j.jep.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Lin HJ, Tseng CP, Lin CF, Liao MH, Chen CM, Kao ST, Cheng JC. A Chinese Herbal Decoction, Modified Yi Guan Jian, Induces Apoptosis in Hepatic Stellate Cells through an ROS-Mediated Mitochondrial/Caspase Pathway. Evid Based Complement Alternat Med. 2011;2011:459–531. doi: 10.1155/2011/459531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 8.Mohamadnejad M, Sohail MA, Watanabe A, Krause DS, Swenson ES, Mehal WZ. Adenosine inhibits chemotaxis and induces hepatocyte-specific genes in bone marrow mesenchymal stem cells. Hepatology. 2010;51:963–973. doi: 10.1002/hep.23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novo E, Busletta C, Bonzo LV, Povero D, Paternostro C, Mareschi K, Ferrero I, David E, Bertolani C, Caligiuri A, et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J Hepatol. 2011;54:964–974. doi: 10.1016/j.jhep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Miyata E, Masuya M, Yoshida S, Nakamura S, Kato K, Sugimoto Y, Shibasaki T, Yamamura K, Ohishi K, Nishii K, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008;111:2427–2435. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
- 11.Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill CK, Kanel GC, DeLeve LD. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137:704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diesselhoff-den Dulk MM, Crofton RW, van Furth R. Origin and kinetics of Kupffer cells during an acute inflammatory response. Immunology. 1979;37:7–14. [PMC free article] [PubMed] [Google Scholar]
- 13.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 14.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Aristizábal A, Keating A, Davies JE. Mesenchymal stromal cells as supportive cells for hepatocytes. Mol Ther. 2009;17:1504–1508. doi: 10.1038/mt.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol. 2006;87:343–359. doi: 10.1111/j.1365-2613.2006.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian YW, Smith PG, Yeoh GC. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Histochem Cell Biol. 1997;107:243–250. doi: 10.1007/s004180050109. [DOI] [PubMed] [Google Scholar]
- 19.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 20.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepard JL, Zon LI. Developmental derivation of embryonic and adult macrophages. Curr Opin Hematol. 2000;7:3–8. doi: 10.1097/00062752-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103–118. doi: 10.1007/s12015-010-9126-5. [DOI] [PubMed] [Google Scholar]
- 24.Kashofer K, Siapati EK, Bonnet D. In vivo formation of unstable heterokaryons after liver damage and hematopoietic stem cell/progenitor transplantation. Stem Cells. 2006;24:1104–1112. doi: 10.1634/stemcells.2005-0405. [DOI] [PubMed] [Google Scholar]
- 25.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Rénia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766–1775. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, Novo E, Bertolani C, Milani S, Vizzutti F, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230–238. doi: 10.1016/j.jhep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Harada K, Chiba M, Okamura A, Hsu M, Sato Y, Igarashi S, Ren XS, Ikeda H, Ohta H, Kasashima S, et al. Monocyte chemoattractant protein-1 derived from biliary innate immunity contributes to hepatic fibrogenesis. J Clin Pathol. 2011;64:660–665. doi: 10.1136/jclinpath-2011-200040. [DOI] [PubMed] [Google Scholar]
- 29.Crane MJ, Hokeness-Antonelli KL, Salazar-Mather TP. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands. J Immunol. 2009;183:2810–2817. doi: 10.4049/jimmunol.0900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thannickal VJ, Toews GB, White ES, Lynch JP, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 31.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Q, Ren H, Zhu D, Han Z. Stem/progenitor cells in liver injury repair and regeneration. Biol Cell. 2009;101:557–571. doi: 10.1042/BC20080105. [DOI] [PubMed] [Google Scholar]


