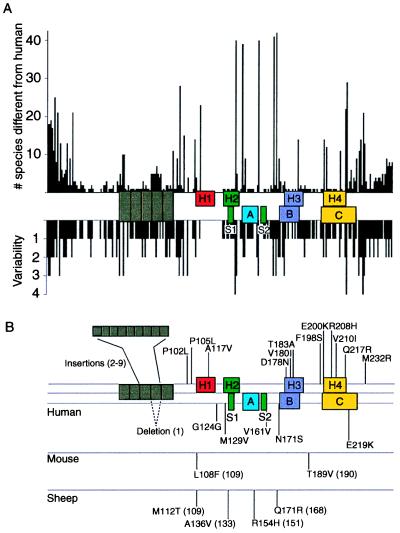
Figure 4.
Species variations and mutations of the prion protein gene. (A) Species variations. The x-axis represents the human PrP sequence, with the five octarepeats and H1–H4 regions of putative secondary structure shown as well as the three α-helices A, B, and C and the two β-strands S1 and S2 as determined by NMR. The precise residues corresponding to each region of secondary structure are given in Fig. 5. Vertical bars above the axis indicate the number of species that differ from the human sequence at each position. Below the axis, the length of the bars indicates the number of alternative amino acids at each position in the alignment. (B) Mutations causing inherited human prion disease and polymorphisms in human, mouse, and sheep. Above the line of the human sequence are mutations that cause prion disease. Below the lines are polymorphisms, some but not all of which are known to influence the onset as well as the phenotype of disease. Data were compiled by Paul Bamborough and Fred E. Cohen.