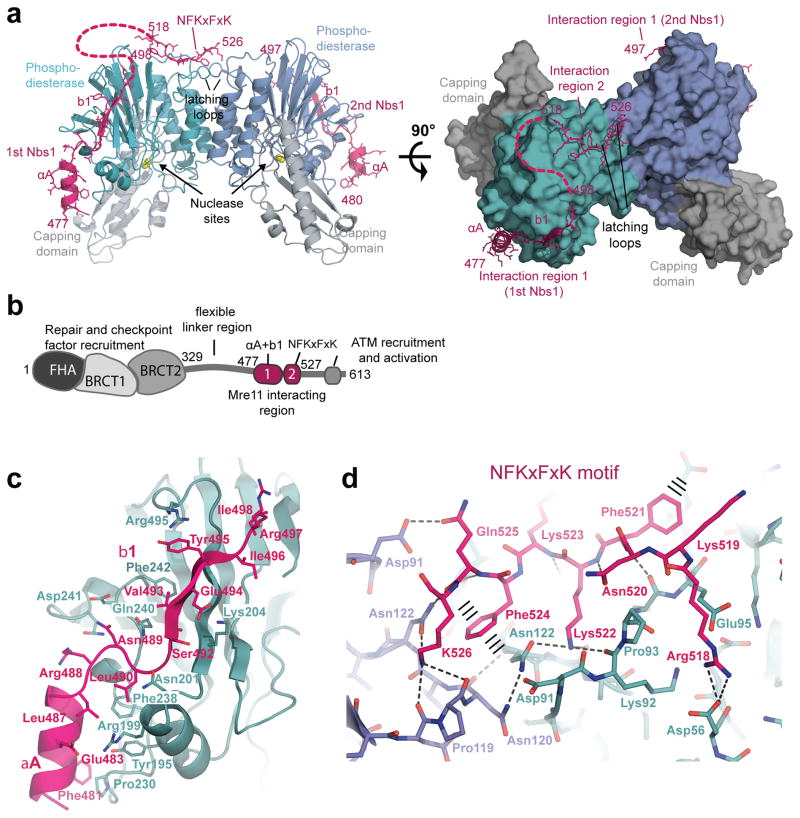
Figure 2. Structure of the Nbs1mir–Mre11cd complex.
(a) Structure of the two Nbs1mir (magenta) bound to the Mre11cd dimer (cyan/blue phosphodiesterase and grey capping domains), shown as ribbon representation with highlighted secondary structures. Nbs1 binds with “interaction region 1” around the outside of the phosphodiesterase domain. One of the two Nbs1 additionally binds with “interaction region 2” to two “signaling loops” at the Mre11 dimer interface.
(b) Molecular surface representation of the Mre11 dimer with bound Nbs1 molecules highlights the asymmetric bridging of the Mre11 dimer by Nbs1 “interaction region 2”.
(c) Domains and motifs of S. pombe Nbs1.
(d) Mre11 interaction region 1 of Nbs1 (magenta) binds to the outside of Mre11’s phosphodiesterase domain (cyan) with two secondary structure elements (αA and b1) and partially polar, partially hydrophobic interface. Key residues from both interaction partners are annotated.
(e) Interaction region 2 of Nbs1 (magenta) contains the highly conserved NFKxFxK motif and binds asymmetrically across the Mre11 dimer (blue/cyan) via a network of hydrogen bonds and π–stacking interactions (highlighted). The structure is rotated by 180° around its central vertical axis in comparison to Figure 2a.