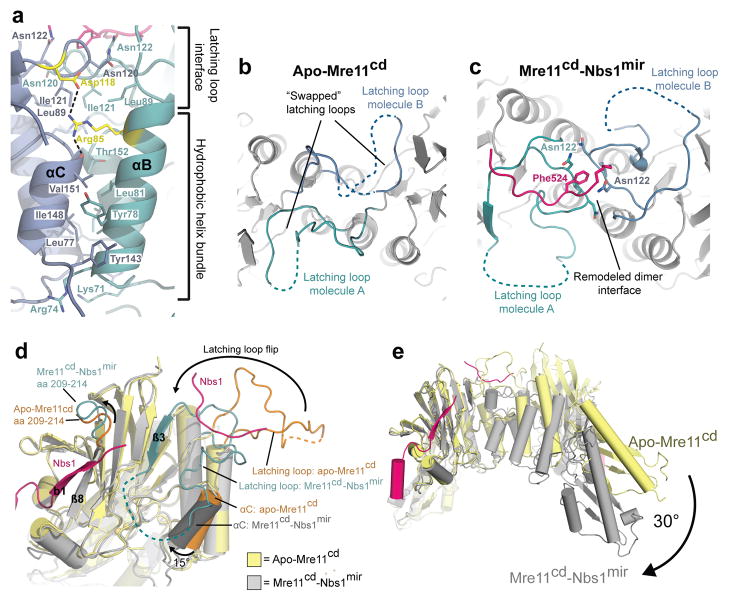
Figure 4. Conformational impact of Nbs1 binding on the Mre11 dimer configuration.
(a) Details of the Mre11 dimer interface in the Nbs1mir–Mre11cd structure. The Mre11 monomers are colored in blue and cyan, while Nbs1mir is colored in pink. The dimer interface can be distinguished into two different regions, the hydrophobic four–helix and the latching loop interface. Both motifs are connected with each other via salt bridges between the conserved residues SpMre11 Arg85 and Asp118 (colored in yellow). The structure is rotated by 180° around its central vertical axis in comparison to Figure 2a.
(b) A top view of apo–Mre11cd along the Mre11 dimer axis shows the latching loops indicated in cyan (molecule A) and blue (molecule B).
(c) A top view of Nbs1mir–Mre11cd similar to (B) on the latching loop conformation in presence of the bound Mre11 interaction region 2 of Nbs1.
(d) Nbs1 binding causes several conformational rearrangements in Mre11. Shown is an overlay of Mre11 monomers from apo–Mre11cd and Nbs1mir–Mre11cd structures.
(e) An overlay of apo–Mre11cd and Nbs1mir–Mre11cd by aligning of Nbs1mir–Mre11cd to just one apo–Mre11cd protomer reveals a distinct macromolecular change. The dimer angle of Nbs1mir–Mre11cd is rotated by 30°, in comparison to apo–Mre11cd, towards a more compact and closed conformation.