Abstract
Objective
To examine the association between prenatal methamphetamine exposure and inhibitory control in 66 month old children followed since birth in the multicenter, longitudinal Infant Development, Environment and Lifestyle Study.
Study design
The sample included 137 children with prenatal methamphetamine exposure and 130 comparison children, matched for race, birth weight, maternal education and type of insurance. Inhibitory control, an executive function related to emotional and cognitive control, was assessed using a computerized Stroop-like task developed for young children. Hierarchical linear modeling tested the relationship between the extent (heavy, some and no use) of prenatal methamphetamine exposure and accuracy and reaction time outcomes, adjusting for prenatal exposure to alcohol, tobacco and marijuana, age, sex, socioeconomic status, caregiver IQ and psychological symptoms, child protective services report of physical or sexual abuse, and site.
Results
In adjusted analyses, heavy prenatal methamphetamine exposure was related to reduced accuracy in both the incongruent and mixed conditions on the Stroop task. Caregiver psychological symptoms and Child Protective Services (CPS) report of physical or sexual abuse were associated with reduced accuracy in the incongruent and mixed, and incongruent conditions, respectively.
Conclusions
Heavy prenatal methamphetamine exposure, along with caregiver psychological distress and child maltreatment, is related to subtle deficits in inhibitory control during the early school-aged years.
Keywords: Prenatal exposure, Amphetamines, Children, Neuropsychology, Executive Function
Methamphetamine use during pregnancy has increased over the past 20 years, with recent estimates suggesting a 5% prevalence in regions with endemic use (1). There is a paucity of research on the developmental consequences of prenatal methamphetamine exposure in children. Like cocaine, methamphetamine is a psychostimulant that blocks dopamine, norepinephrine and serotonin reuptake, increasing concentrations of these neurotransmitters in the synaptic cleft (2). Methamphetamine also enhances release of these neurotransmitters, inhibits monoamine oxidase, and causes maternal vasoconstrictive and anorectic effects (3). Prenatal methamphetamine exposure may additionally impact widespread neuro-ontogenic processes, such as cell production and migration (4), alter development of the fetal stress response axis (5), and perturb oxidative, mitochondrial and glutamate-associated excitotoxic pathways leading to neuronal damage (6).
Prenatal methamphetamine exposure has been linked to deficits in fetal growth (7) and to effects on infant arousal-regulation, stress reactivity, and motor control (8, 9), which could increase the risk for later problems in cognitive, psychomotor, and behavioral functioning (10–12).
Prenatal methamphetamine exposure may also be associated with deficits in higher order executive functions that are considered foundational for academic, psychosocial and behavioral function during later childhood and adolescence (13, 14). Neuroimaging studies of community-derived convenience samples (15–17) have identified alterations in frontal-striatal brain regions thought to be related to specific executive function such as inhibitory control, working memory, sustained attention, and visual-motor integration (18). Of these skills, inhibitory control, the ability to resist a first impulse or to stay on task despite distraction (19), is considered to be particularly important for the development of social competence (20), emotional and cognitive control (21). inhibitory control deficits have been reported in prospective, longitudinal studies of children exposed prenatally to cocaine (21–25). Here, we report relationships between prenatal methamphetamine exposure and inhibitory control at 66 months of age among children enrolled in the large prospective study of prenatal methamphetamine exposure. We hypothesized that prenatal methamphetamine exposure would be associated with poorer inhibitory control, and that children with heavier prenatal exposure would have more pronounced deficits.
METHODS
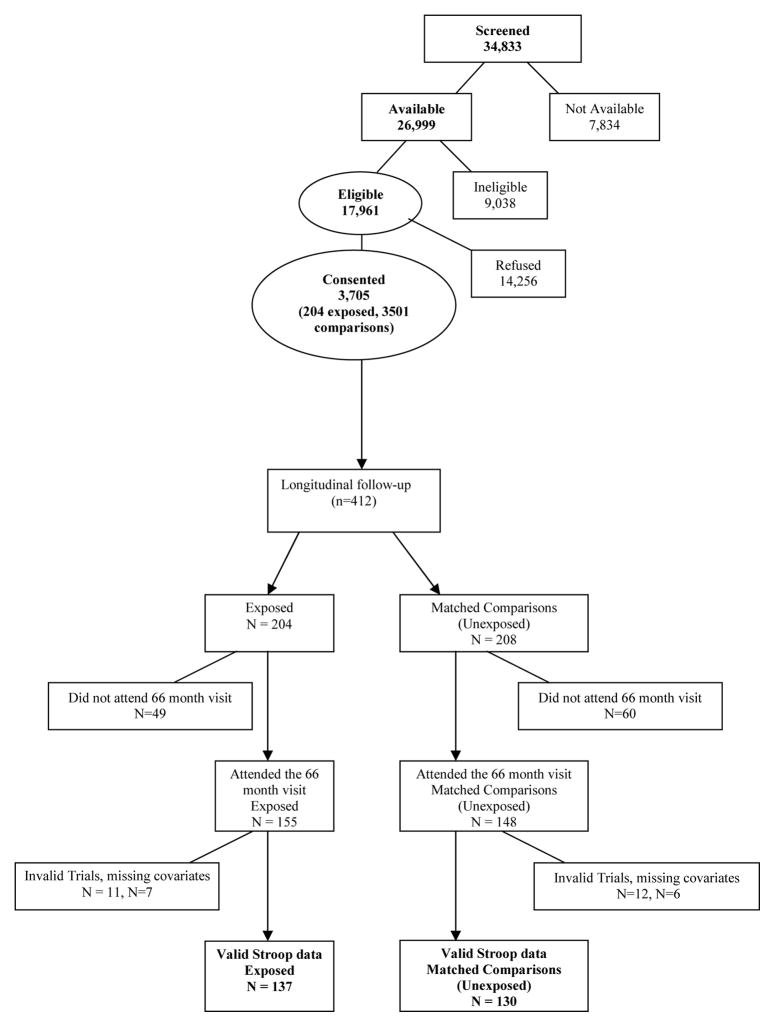
Mothers and their infants were enrolled at birth in the longitudinal Infant Development, Environment and Lifestyle (IDEAL) study of prenatal methamphetamine exposure, conducted at five clinical sites in geographic areas with high methamphetamine use – the University of California, Los Angeles; the University of Hawaii; Blank Children’s Hospital-Iowa Health; and the Universities of Oklahoma and Tulsa. Institutional Review Board approval was obtained at each site and included a federal Certificate of Confidentiality. Detailed recruitment methods have been reported previously (1, 26). Maternal exclusion criteria were age <18, opiate use during pregnancy, institutionalization for retardation or emotional disorders, overt psychosis or a documented history of psychosis, and inability to speak English. Infants exclusion criteria were critical illness (unlikely to survive), multiple birth, major life-threatening congenital anomaly, documented chromosomal abnormality associated with mental or neurologic deficiency, overt infection, and having a sibling previously enrolled in the IDEAL study (Figure). Between September 2002 and November 2004, 34,833 women delivering at the above sites were screened, of which 26,999 were available and 17,961 eligible for participation. The most common reason for ineligibility was having a non-English speaking mother. Of the eligible mothers, 3705 consented and 14,256 refused. The 21% consent rate is consistent with other studies of this kind (1). Sociodemographic and substance use information was collected from maternal interviews including the Lifestyle Interview and Substance Use Inventory. Meconium samples were collected from all infants and analyzed by a central laboratory (US Drug Testing Laboratory, Des Plaines, IL) for drug metabolites. Methamphetamine exposure was determined by self-report and/or a positive meconium screen with Gas Chromatography/Mass Spectroscopy confirmation.
Figure 1.
Flow chart of subject recruitment and enrollment in the current study.
For longitudinal follow up, infants prenatally exposed to methamphetamine and mothers (n=204) were matched to unexposed comparison infant- mother pairs (n=208) who denied methamphetamine use and had a negative meconium screen. The two groups were matched for maternal race, birth weight category (<1500 g, 1500–2500 g, >2500 g), private versus public insurance, and education (high school education completed versus not completed). Prenatal exposure to alcohol, tobacco and marijuana existed in both groups and were considered as background variables. Follow-up assessments were conducted at 1, 12, 24, 30, 36, 60 and 66 months of age.
Measures
Inhibitory Control
Executive function at the 66-month-visit was measured with the Hearts and Flowers version of the Dots task from the Directional Stroop Battery for school age children (27). This task tests both inhibitory control and working memory, but in younger children the task demands for inhibitory control is thought to exert a stronger effect on performance than the memory demands (27). Certified examiners masked to exposure status administered the task (19), which was conducted on a laptop computer with children seated approximately 53 cm from the 19 cm × 30 cm computer screen. During each trial, a red heart or a red flower was presented on the left or right side of the computer screen and subjects were instructed to press either the left or right green-labeled “shift” keys in response to the stimulus, depending on the rule, described in more detail below. The trial sequence of events was as follows: plus sign centered on the computer screen (500 msec); blank screen (500 msec); heart or flower presentation (1500 msec or less if child responds during the interval); blank screen for 500 msec. The interstimulus interval was 1500 msec. The maximum trial duration was 3000 msec. The allowable response time from onset of the stimulus is 2000 msec. There were three task conditions (congruent, incongruent, mixed) administered in sequential blocks of trials. Prior to the first two conditions, the child practiced the rule with 4 trials that were identical to the task except the stimulus remained on the screen until the button was pressed and the child was given feedback and allowed to self correct. If a subject missed 2 out of the 4 practice trials, additional practice sets, up to three in total, were automatically run.
In the congruent condition (first block with 12 trials), the child followed the rule, “press the button on the same side as the heart”. In the more difficult incongruent condition (second block with 12 trials), the child followed the rule, “press the button on the side opposite the flower”. In the most challenging mixed condition with randomly intermixed congruent and incongruent trials (third block of 33 trials), the child had to hold two rules in mind, “hearts means same side and flowers means opposite side”. Each subject was required to complete all three conditions of the Hearts and Flowers task to be included in the study. No corrective feedback was given to the child during test trials. Performance on the task was assessed by: (1) percentage of correct responses or accuracy, measured by dividing correct responses by correct + incorrect responses; and (2) reaction time, calculated as the mean for correct responses only. Accuracy rather than speed is thought to be a more sensitive measure for young children using the incongruent Hearts and Flowers test (28), and from preschool through school age, to adulthood, accuracy measures tend to fit the following pattern: congruent > incongruent ≫ mixed (27).
The task was administered to 303 children (74% of the full sample of 412 participating in the 66-month visit) (Figure); 23 participants were excluded due to ≥ 50% invalid trials on any of the three trials blocks of the task. Trials were invalid if the child did not respond within 2,000 msec or pressed the response key in ≤200 msec, indicating either non-physiologically possible anticipatory guessing, or failure to release the response button from a prior trial. Of those 23 participants, eleven cases were excluded due to persistent non-responding, 8 cases for anticipatory responses, and 4 cases for both. In addition 13 participants were excluded due to missing data for covariates. Thus, there were 267 children with valid, complete data (n=137 infants prenatally exposed to methamphetamine and n=130 comparison). The median age of administration was 66 months with 90% ± 6 months with no differences in age by exposure status (p=0.570). Age at assessment was included as a covariate in analysis of executive function. Comparing the 36 children excluded due to invalid trials or missing data with the 267 who completed the task, no differences were found in prenatal methamphetamine exposure or other drugs, sex, or Bayley Scales of Infant Development-II mental or psychomotor development scores at 24 or 36 months.
Covariates
At recruitment, demographic and neonatal characteristics were obtained from the Lifestyle Interview including race, sex, insurance (public or private), maternal age, having a partner (coded as yes/no), socioeconomic status (SES) and neonatal growth (birth weight, length, head circumference and gestational age). SES was calculated using the 4-factor Hollingshead Index adapted for single parent and nonnuclear families (29, 30). For descriptive purposes, low SES (Hollingshead V) at recruitment is reported in Tables I and II. Prenatal use of methamphetamine and other drugs including the quantity and frequency of use was obtained from the Substance Use Inventory (31). Consistent with other published studies (9, 32), heavy methamphetamine use was defined as ≥ 3 days per week across pregnancy. Some use was any use not meeting the criterion for heavy use. Postnatal caregiver and environmental characteristics were measured on multiple visits and averaged or aggregated for this study. Measures from the Lifestyle Interview administered at 1, 12, 24, 36, 60 and 66 months included change in primary caregiver (yes/no), child protective services (CPS) report of physical or sexual abuse (yes/no), and SES (index of social position). The Substance Use Inventory at 12, 24, 36, 60, and 66 months assessed postnatal caregiver use of methamphetamine, alcohol, tobacco and marijuana (yes/no for each drug) (31). The personal safety section of the Substance Use Inventory at 36 and 66 months assessed domestic violence for any physical or sexual abuse (yes/no). The Brief Symptom Inventory (BSI), a 53-item questionnaire administered at 1, 12 and 36 months, yielded an overall score of caregiver psychological symptoms (33). Caregiver depression was assessed by the Beck Depression Inventory-II at 1, 12 and 36 months (34). Caregiver receptive vocabulary, a proxy measure of caregiver intelligence quotient, was assessed by the Peabody Picture Vocabulary Test – 3rd edition at the 30-month home visit (35). The quality of the home environment, computed as an overall summary score, was measured at 30 months of age using the Early Childhood Home Observation for Measurement of the Environment (HOME) Inventory (36).
Table 1.
Maternal and infant characteristics at birth by level of prenatal methamphetamine exposure
| Maternal/demographic characteristics | Heavy PMAE | Some PMAE | No PMAE | p |
|---|---|---|---|---|
| Number (%)/Mean (SD) | (n=26) | (n=107) | (n=130) | |
| Ethnicity | 0.971 | |||
| White | 13 (50.0%) | 39 (36.4%) | 55 (42.3%) | |
| Hispanic | 4 (15.4%) | 23 (21.5%) | 28 (21.5%) | |
| Pacific Islander | 4 (15.4%) | 20 (18.7%) | 20 (15.4%) | |
| Asian | 4 (15.4%) | 15 (14.0%) | 17 (13.1%) | |
| Black | 1 (3.8%) | 6 (5.6%) | 7 (5.4%) | |
| American Indian | 0 (0%) | 4 (3.7%) | 3 (2.3%) | |
| Low SES | 12 (46.2%) | 34 (31.8%) | 10 (7.7%) | <.001 |
| Public insurance | 24 (92.3%) | 95 (88.8%) | 112 (86.2%) | 0.216 |
| No partner | 17 (65.4 %) | 61 (57.0%) | 38 (29.2%) | <.001 |
| Education <12 years | 16 (61.5%) | 47 (43.9%) | 44 (33.8%) | 0.024 |
| Maternal age (year) | 25.9 ± 5.9 | 25.2 ± 5.4 | 24.8 ± 5.7 | 0.633 |
| Prenatal alcohol use | 7 (26.9%) | 36 (33.6%) | 18 (13.8%) | <.001 |
| Alcohol heavy use (≥ 0.5 oz absolute alcohol/day across pregnancy) | 4 (3.8%) | 0 | - | |
| Prenatal marijuana use | 8 (30.8%) | 33 (30.8%) | 6 (4.6%) | <.001 |
| Marijuana heavy use (≥ 0.5 joints/day across pregnancy) | 3 (2.8%) | 2 (1.5%) | 0.674 | |
| Prenatal tobacco use | 23 (88.5%) | 85 (79.4%) | 35 (26.9%) | <.001 |
| Tobacco heavy use (≥10 cigarettes/day across pregnancy) | 15 (57.7%) | 27 (25.2%) | 6 (4.6%) | <.001 |
| Infant characteristics | ||||
| Sex, male | 17 (65.4%) | 54 (50.5%) | 67 (51.5%) | 0.376 |
| Birth weight (g) | 3274 ± 649 | 3189 ± 638 | 3268 ± 542 | 0.557 |
| Gestational age (wk) | 38.7 ± 1.9 | 38.1 ± 2.4 | 39.0 ± 1.8 | 0.006 |
| Low birth weight | 1 (3.8%) | 15 (14.0%) | 15 (11.5%) | 0.350 |
| Length (cm) | 50.5 ± 3.4 | 49.9 ± 3.7 | 50.9 ± 3.1 | 0.053 |
| Head circumference (cm) | 33.9 ± 1.9 | 33.7 ± 1.9 | 33.9 ± 1.8 | 0.470 |
PMAE, prenatal methamphetamine exposure
Note: 4 cases do not have level of PMAE due to identification of exposure by meconium only
Table 2.
Postnatal characteristics of the environment by level of prenatal methamphetamine exposure
| Number (%)/Mean (SD) | Heavy PMAE | Some PMAE | No PMAE | p |
|---|---|---|---|---|
| (n=26) | (n=107) | (n=130) | ||
| Caregiver Change (by 66 months of age) | 19 (73.1%) | 60 (56.1%) | 13 (10.0%) | <0.001 |
| Postnatal Tobacco Use (by 66 months of age) | 15 (57.7%) | 67 (63.8%) | 63 (49.2%) | 0.081 |
| Postnatal Alcohol Use (by 66 months of age) | 19 (73.1%) | 70 (66.0%) | 94 (72.9%) | 0.493 |
| Postnatal Marijuana Use (by 66 months of age) | 3 (12.0%) | 17 (16.3%) | 12 (9.4%) | 0.287 |
| Domestic Violence (by 66 months of age) | 2 (7.7%) | 10 (9.3%) | 4 (3.1%) | 0.128 |
| Average Caregiver Psychological Symptoms (at 1, 12, and 36 months) | 0.47 (0.40) | 0.46 (0.41) | 0.43 (0.40) | 0.853 |
| Average Caregiver Depression (at 1, 12, and 36months) | 9.1 (7.2) | 9.4 (7.6) | 8.6 (6.0) | 0.661 |
| Caregiver Receptive Vocabulary (at 30 months) | 94.4 (10.5) | 91.1 (11.9) | 90.8 (13.8) | 0.416 |
| Quality of Home (HOME score at 30 months) | 34.8 (3.4) | 34.0 (3.9) | 34.6 (3.7) | 0.494 |
| Child Protective Services report of physical or sexual abuse (by 66 months of age) | 3 (11.5%) | 7 (6.5%) | 4 (3.1%) | 0.164 |
Note: 4 cases do not have level of PMAE due to identification of exposure by meconium only
Data Analysis
Analysis of variance was used to compare means for continuous variables and Chi-square tests to compare proportions for categorical variables. General linear models were applied to the level of methamphetamine use to compare heavy use versus no use and some use versus no use. Hierarchical linear models (HLM, SAS Proc MIXED, version 9.1.3, Cary, NC) were used to test associations between level of prenatal methamphetamine exposure (heavy, some, no use) and each of the six accuracy and reaction time outcomes. In multivariate analysis, we adjusted for covariates in each model. Continuous covariates (e.g., age at assessment) were grand mean centered. Pearson correlations were used to test associations between accuracy and reaction times in each of the three conditions of the Hearts and Flowers task, and to follow up on significant covariate effects.
A priori covariates included prenatal exposure to alcohol, tobacco, and marijuana, sex, SES at birth, age at assessment, study site, and SES averaged through 66 months. Participant characteristics that differed between methamphetamine exposure groups (P<0.05) were included if not highly (r<0.7) correlated with other covariates. Covariates measured at multiple time points were averaged (e.g., SES, caregiver psychological symptoms) or aggregated over time (e.g., any caregiver postnatal tobacco use 12 to 66 months). Interactions between prenatal methamphetamine exposure and covariates were tested and removed if p>.10. Final covariates were selected only if the p value overall or any category-wise was ≤. 10. To provide uniformity across analyses, covariates that met criteria for one outcome were retained in all models. The final covariate set included prenatal exposure to alcohol, tobacco, and marijuana, age at assessment, sex, SES, caregiver IQ, CPS report of physical or sexual abuse, caregiver psychological symptoms and site. The prenatal drug exposures were continuous measures of these drugs (oz absolute alcohol/day, average number cigarettes/day and average number joints/day across pregnancy).
RESULTS
The sample (N=267) for this study is 65% of the original sample recruited (n=412). To assess selective attrition, we compared child and caregiver characteristics of participants included in this study (n=267) with those not included (n=145). There were fewer heavy alcohol and marijuana users in the included group.
Table I shows maternal and infant characteristics at birth by heavy, some and no prenatal methamphetamine exposure. Caregivers in the heavy prenatal methamphetamine exposure group were more likely to be low SES, not have a partner or a high school education, and have used more marijuana and tobacco, including heavy tobacco use, than no prenatal methamphetamine exposure group. Some prenatal methamphetamine exposure was associated with lower gestational age than no prenatal methamphetamine exposure. Caregivers in the some prenatal methamphetamine exposure group were more likely to be low SES, not have a partner, and have used more, alcohol, marijuana and tobacco, including heavy tobacco use, than caregivers in the group with no prenatal methamphetamine exposure.
In the postnatal period through 66 months, the heavy and some prenatal methamphetamine exposure groups were more likely to have a caregiver change due in part to mandatory reporting of illicit drug use during pregnancy to CPS, frequently resulting in child removal (Table II).
Table III shows results for accuracy and reaction time by level of prenatal methamphetamine exposure. In both unadjusted and adjusted analyses, heavy prenatal methamphetamine exposure, but not some prenatal methamphetamine exposure, was associated with less accuracy in the incongruent and mixed conditions.
Table 3.
Accuracy and reaction time for congruent, incongruent and mixed conditions by level of prenatal methamphetamine exposure
| Outcome | Level of PMAE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heavy (h) (N=25) |
Some (s) (N=105) |
No (n) (N=130) |
Adjusted a | |||||||
| β | 95% CI | β | 95% CI | |||||||
| % Correct | Mean | SD | Mean | SD | Mean | SD | h vs. n | h vs. n | s vs. n | s vs. n |
| Congruent | 91.0 | 9.8 | 87.6 | 19.7 | 90.9 | 16.6 | −1.86 | (−10.4, 6.7) | −3.82 | (−8.6, 1.0) |
| Incongruent | 54.4 | 31.3 | 70.1 | 26.9 | 74.6 | 26.9 | −22.69 | (−35.9, −9.4) | −4.29 | (−11.7, 3.1) |
| Mixed | 57.9 | 14.7 | 63.6 | 17.7 | 67.8 | 18.5 | −8.79 | (−17.3, −0.3) | −3.52 | (−8.3, 1.2) |
| Mean reaction time (msec) | ||||||||||
| Congruent b | 748 | 230 | 749 | 242 | 773 | 262 | −99.55 | (−218.2, 19.1) | −59.85 | (−126.2, 6.5) |
| Incongruent b | 910 | 253 | 900 | 235 | 941 | 267 | −22.89 | (−157.1, 111.3) | −45.72 | (−117.9, 26.5) |
| Mixed | 985 | 252 | 1030 | 246 | 1049 | 254 | −79.51 | (−199.7, 40.7) | −28.36 | (−95.9, 39.2) |
Note: 4 cases do not have level of PMAE due to identification of exposure by meconium only; 3 cases do not have level of alcohol, tobacco or marijuana exposure.
All analyses adjusted for absolute alcohol/d, cigarettes/d, and joints/d during pregnancy, age at assessment, sex, SES (avg. birth to 66 months), caregiver IQ, CPS report of physical or sexual abuse (any report through 66 months), caregiver psychological symptoms (avg 1, 12 and 36 months).
1case (h) in the congruent condition and 10 cases (3 h, 3 s, 4 n) in the incongruent condition had no correct responses, hence missing reaction time for correct response. No association with level of PMAE.
In adjusted analyses, quantity of prenatal tobacco use was associated with longer reaction time in the congruent condition (P=0.027). Caregiver IQ was associated with greater accuracy in the congruent and mixed conditions (all P’s<0.01). Caregiver psychological symptoms were associated with less accuracy in the incongruent and mixed conditions (all P’s<0.03), longer reaction time in the congruent condition but decreased reaction time in the mixed condition (all P’s<0.05). CPS reports of physical or sexual abuse was associated with reduced accuracy in the incongruent condition (P=.034).
DISCUSSION
Prenatal methamphetamine exposure is associated in a dose response manner with deficits in laboratory measures of inhibitory control in the early school age period, after adjusting for key covariates. These executive function differences were found despite no differences by prenatal methamphetamine exposure or heavy prenatal methamphetamine exposure status in standardized assessments of mental or psychomotor development at 12, 24 and 36 months (32) or in language and behavioral scores at 36 months (38).
Our findings of reduced inhibitory control performance in children with prenatal methamphetamine exposure are consistent with earlier theoretical work linking sympathomimetic drugs like cocaine and methamphetamine to altered prefrontal cortex development and function (39), with studies in children (40) and non-human primates that provide evidence at the neural level that frontal-subcortical circuits are critical for successful manipulation of situations where there is a response conflict (41), and with recent neuroimaging studies of prenatal methamphetamine exposure that have identified alterations in frontal-striatal circuitry, as reflected by changes in white matter diffusivity (42), neurometabolite concentrations (16), and fMRI brain activation during executive function tasks (17).
Our findings are similar to prospective studies that reported deficits in inhibitory control after prenatal exposure to cocaine (21–25), one of which (22) also found effects associated with higher average diffusion measures in frontal brain regions, suggesting lower integrity or slower maturation of cortical white matter fiber tracts. A recent summary of school-age studies of children with prenatal cocaine exposure suggested compromised performance in sustained attention and behavioral self-regulation (43), skills that are highly related to inhibitory control development.
We also found associations between inhibitory control and both caregiver psychological distress, as measured by the BSI, and cumulative child physical and/or sexual abuse as reported by CPS. Previous research has linked early supportive caregiving with the development of inhibitory control (44), so it is not unexpected that caregiver distress and maltreatment might relate to poorer inhibitory control function, possibly mediated through HPA axis dysregulation and compromised prefrontal cortex development (45, 46). That these associations were independent of the main effect of prenatal methamphetamine exposure on inhibitory control provides additional evidence in support of a growing body of research linking early socio-environmental adversity, both with and without preceding prenatal drug exposure, with inhibitory control deficits (47–49) and with the later appearance in adolescence of a more complex disinhibitory phenotype (50). However, because caregiver psychological distress was measured several years antecedent to the inhibitory control outcome, it may be unmeasured concurrent exposure to caregiver distress rather than earlier exposure that is driving this association. It is possible that the findings of reduced inhibitory control among children with prenatal methamphetamine exposure may reflect the post-natal effects of cumulative early adversity conditions (i.e. low SES, high caregiver turnover, caregiver psychopathology, household violence, etc.) on the development of childhood mental health, cognitive and psychosocial impairments (51, 52). It is also possible that the poorer inhibitory control observed in the prenatal methamphetamine exposure cohort was related to early caregiver instability, because by 60 months of age 60% of the exposed group was no longer living with their family of origin. Two recent studies provide support for this link between placement instability and children’s inhibitory control (47), even after controlling for neglect (45).
There are several limitations of the present study. First, the follow-up rate of 65% in this study, although typical of studies involving high risk cohorts, raises the possibility of retention bias; however, the evaluated and not-evaluated groups differed only in regard to number of heavy alcohol and marijuana users, suggesting that our results are conservative, because heavy use of these drugs occurs predominantly in mothers using methamphetamine. Second, the relatively small number of heavy methamphetamine users implies that our results be considered preliminary. Third, although the multivariate analyses adjusted for prenatal exposure to alcohol, tobacco and marijuana, allowing identification of outcomes associated independently with heavy prenatal methamphetamine exposure, it is possible that residual confounding, multiple additive drug exposures, or drug-drug interactions explain some of the findings. Fourth, the association between cumulative physical and/or sexual abuse as reported by CPS at 66 months of age and inhibitory control deficits, although consistent with other research on neglected or maltreated children, needs to be taken with caution, given the small numbers of children in both exposure groups (prenatal methamphetamine exposure = 11; no prenatal methamphetamine exposure = 4) with identified abuse. And lastly, intergenerational transmission of a genetic predisposition to inhibitory control deficits and coexistent substance use disorder, rather than a potential neuroteratologic mechanism, may explain the observed associations between prenatal methamphetamine exposure, socio-environmental adversity, and children’s inhibitory control.
Acknowledgments
Supported by National Institutes on Drug Abuse (grants 2R01DA014948 [to B.L.] and 1K23DA020801 [to C.D.]) and, in part, by the National Center for Research Resources (5P20RR11091 and 3M01RR00425).
We would like to sincerely thank the children, families, and staff participating in the IDEAL Study.
Abbreviations
- BSI
Brief Symptom Inventory
- HOME
Home Observation for Measurement of the Environment
- NIDA
National Institutes on Drug Abuse
- SES
Socioeconomic Status
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arria A, Derauf C, LaGasse L, Grant P, Shah R, Smith L, et al. Methamphetamine and Other Substance Use During Pregnancy: Preliminary Estimates from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 2.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clin Perinatol. 2009;36:595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Brain Res Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 5.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 6.Tata DA, Yamamoto BK. Interactions between methamphetamine and environmental stress: role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction. 2007;102(Suppl 1):49–60. doi: 10.1111/j.1360-0443.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen D, Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, et al. Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. J Pediatr. 2010;157:337–9. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 2011;33:166–75. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li-Grining CP. Effortful control among low-income preschoolers in three cities: stability, change, and individual differences. Dev Psychol. 2007;43:208–21. doi: 10.1037/0012-1649.43.1.208. [DOI] [PubMed] [Google Scholar]
- 11.Messinger DS, Bauer CR, Das A, Seifer R, Lester BM, Lagasse LL, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113:1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125:e90–8. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 14.Shing YL, Lindenberger U, Diamond A, Li S, Davidson MC. Memory maintenance and inhibitory control differentiate from early childhood to adolescence. Dev Neuropsychol. 2010;35:679–697. doi: 10.1080/87565641.2010.508546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O’Connor MJ, et al. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: The effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54:3067–75. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, et al. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–38. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. [Accessed: 2/17/2011];Science. 2007 318:1387–1388. doi: 10.1126/science.1151148. Supplemental Online Material. http://www.sciencemag.org/content/318/5855/1387/suppl/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengua LJ. Associations among emotionality, self-regulation, adjustment problems, and positive adjustment in middle childhood. Appl Dev Psychol. 2003;24:595–618. [Google Scholar]
- 21.Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33:47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–24. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, et al. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31:159–168. doi: 10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of Prenatal Cocaine Exposure on Attention and Response Inhibition as Assessed by Continuous Performance Tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–351. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 27.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond A, Kirkham N. Not quite as grown-up as we like to think: parallels between cognition in childhood and adulthood. Psychol Sci. 2005;16:291–7. doi: 10.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- 30.LaGasse L, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, et al. The Maternal Lifestyle Study (MLS): the caretaking environment of infants exposed to cocaine/opiates [abstract] Pediatr Res. 1999;45:247A. [Google Scholar]
- 31.Della Grotta S, Lagasse LL, Arria AM, Derauf C, Grant P, Smith LM, et al. Patterns of Methamphetamine Use During Pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2010;14:519–27. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Haning W, et al. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 2011;33:176–84. doi: 10.1016/j.ntt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derogatis LR. BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual. 4. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. The Beck Depression Inventory manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35.Dunn LM, Dunn LM. The Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 36.Caldwell BM, Bradley RH. Little Rock. AR: U of Arkansas; 1984. Home observation for measurement of the environment. [Google Scholar]
- 37.Mickey RM, Dunn OJ, Clark VA. Applied Statistics: Analysis of Variance and Regression. 3. Wiley-Interscience; 2004. [Google Scholar]
- 38.Derauf C, Lagasse L, Smith L, Newman E, Shah R, Arria A, et al. Infant Temperament and High-Risk Environment Relate to Behavior Problems and Language in Toddlers. J Dev Behav Pediatr. 2011;32:125–135. doi: 10.1097/DBP.0b013e31820839d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24:385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 40.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Sci. 2002;5:F9–F16. [Google Scholar]
- 41.Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol Clin Exp Res. 2003;27:647–56. doi: 10.1097/01.ALC.0000060525.10536.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–75. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Dev Psychol. 2000;36:220–32. [PubMed] [Google Scholar]
- 45.Lewis EE, Dozier M, Ackerman J, Sepulveda-Kozakowski S. The effect of placement instability on adopted children’s inhibitory control abilities and oppositional behavior. Dev Psychol. 2007;43:1415–27. doi: 10.1037/0012-1649.43.6.1415. [DOI] [PubMed] [Google Scholar]
- 46.Lester BM, Lagasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, et al. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J Pediatr. 2010;157:288–295.e1. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pears KC, Fisher PA, Bruce J, Kim HK, Yoerger K. Early elementary school adjustment of maltreated children in foster care: the roles of inhibitory control and caregiver involvement. Child Dev. 2010;81:1550–64. doi: 10.1111/j.1467-8624.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valiente C, Lemery-Chalfant K, Reiser M. Pathways to problem behaviors: chaotic homes, parent and child effortful control, and parenting. Social Development. 2007;16:249–267. [Google Scholar]
- 49.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, et al. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Dev Psychopathol. 2011;23:777–88. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutter M, Cox A, Tupling C, Berger M, Yule W. Attainment and adjustment in two geographical areas. I--The prevalence of psychiatric disorder. Br J Psychiatry. 1975;126:493–509. doi: 10.1192/bjp.126.6.493. [DOI] [PubMed] [Google Scholar]
- 52.Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, et al. Family-environment risk factors for attention-deficit hyperactivity disorder. A test of Rutter’s indicators of adversity. Arch Gen Psychiatry. 1995;52:464–70. doi: 10.1001/archpsyc.1995.03950180050007. [DOI] [PubMed] [Google Scholar]