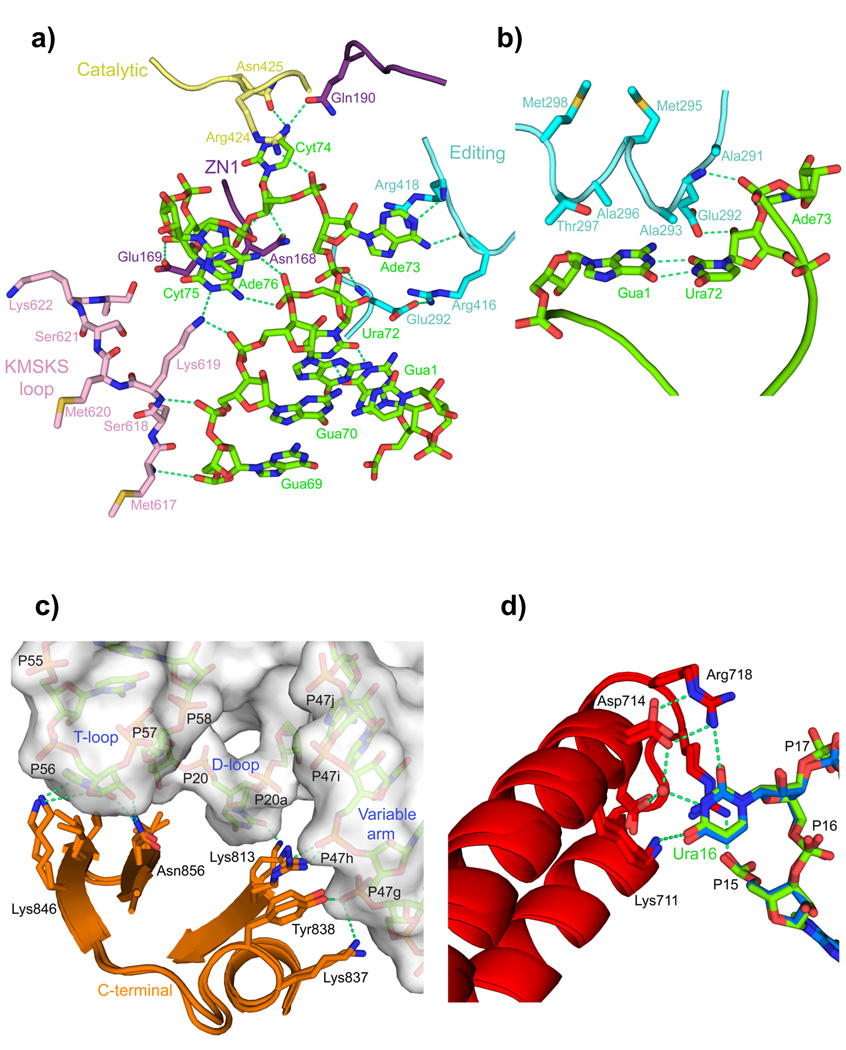
Figure 2. LeuRS-tRNALeu interactions in the aminoacylation complex.
a. Several domains (color coded as in Fig. 1) of LeuRS are involved in binding and stabilizing the conformation of nucleotides 69 to 76 of the 3′ end of tRNALeu (green). The base of Gua71 is omitted for clarity.
b. The α-helix 291–298 of the editing domain stacks on the G1-U72 base pair and contacts the backbone of Ade73.
c. Interactions of the C-terminal domain with the T-loop, D-loop and long-variable arm of the tRNA (surface representation) are conserved in the editing and aminoacylation states (overlaid).
d. A network of interactions from the anti-codon binding domain, conserved between the two states (overlaid), specifically recognizes the base of Ura16 (see also Supplementary Figs. 4a,b).