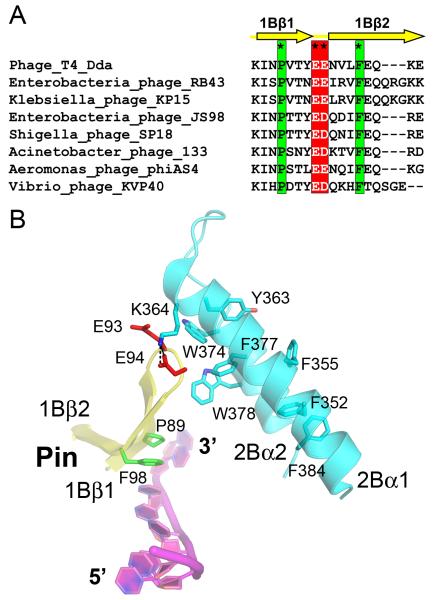
Figure 2. Details of the interaction between the pin and the tower in the Dda-ssDNA complex.
(A) The pin residues are highly conserved in Dda proteins from T4-like phages. Completely conserved is a pair of acidic residues within the tight turn at the end of the pin (asterisks and red box) that mediate an electrostatic interaction with the tower. Also completely conserved are a proline and a phenylalanine (asterisks and green box) that form a stacking interaction with a base of the translocating ssDNA. (B) A close up of the pin-tower interface highlighting key charged and aromatic residues described in the text. E93, E94, K364 and W378 are the residues that have been mutated to probe the functional importance of this interface. In the figure, the pin is shown in yellow, the tower is cyan and the bound ssDNA is magenta.