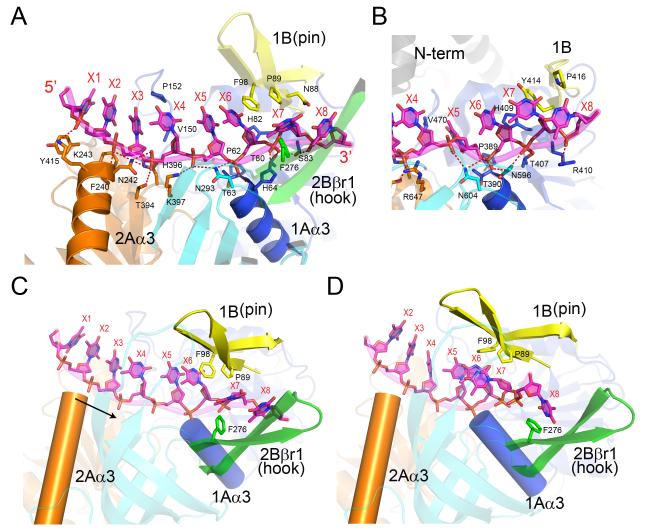
Figure 3. The ssDNA-binding interactions in Dda.
(A) The ssDNA traverses the RecA-like domains 1A and 2A, and P89 and F98 form a triple stack interaction with the DNA base at position X6. Note that the N-termini of helices 1Aα3 (domain 1A) and 2Aα3 (domain 2A) point directly at the DNA 5′ phosphates at positions X6 and X3, respectively. The coloring scheme matches that shown in Figure 1. (B) The open conformation of the RecD2-ssDNA complex (Saikrishnan et al., 2009) centered on position X6. To facilitate the comparison with (A), the two structures are aligned and equivalently colored and labeled. Note that the bases X5, X6 and X7 are in different orientations in the two structures with X5 stacked onto X6 in Dda but rotated into a pocket in RecD2. (C) The observed open conformation structure of the Dda-ssDNA complex highlighting the locations of the pin (yellow), the hook (green), and helices 1Aα3 (blue cylinder) and 2Aα3 (orange cylinder) relative to ssDNA. The arrow indicates the direction in which α-helix 2Aα3 moves relative to ssDNA during domain closure. (D) A model of the closed Dda-ssDNA complex based on the structure of the RecD2 ternary complex (Saikrishnan et al., 2009). Due to domain closure and rotation, Phe98 no longer stacks onto the base at X6, and the hook becomes aligned along the axis of the ssDNA towards the incoming duplex with Phe276 forming a new stacking interaction with the base at position X8. Note that helix 2Aα3 has moved closer to helix 1Aα3 by one phosphate group compared to (C).