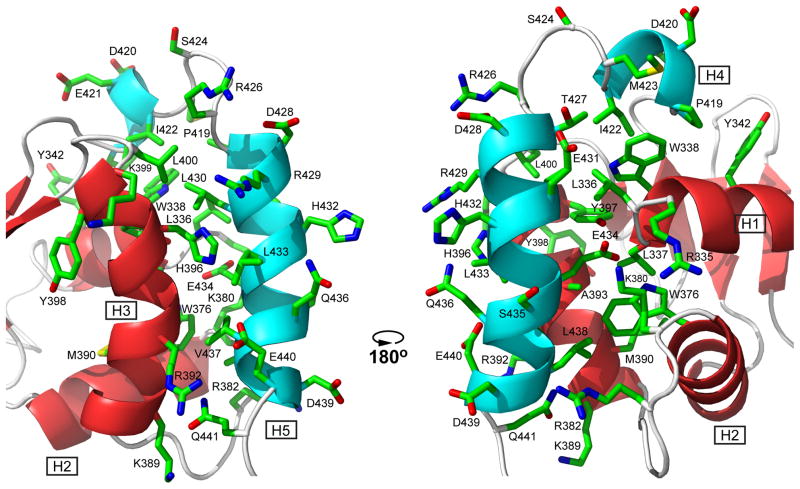
Fig. 3. Helices H4 and H5 of the CID dock along helices H1, H2, and H3 of the ETS domain via hydrophobic and electrostatic interactions.
Shown are the sidechains of all CID residues and selected ETS domain residues in one member of the ETV6R458 structural ensemble (carbon, green; oxygen, red; nitrogen, blue; hydrogens removed for clarity). In this position, the CID sterically blocks the DNA-binding interface of the ETS domain, which is centered around the recognition helix H3 (see Fig. 7) and includes the N-terminus of helix H1 and residues in the turn between helices H2 and H3. Cartoons of the helices and strands are colored as in Fig. 2. The two views are rotated by ~180° about the vertical axis.