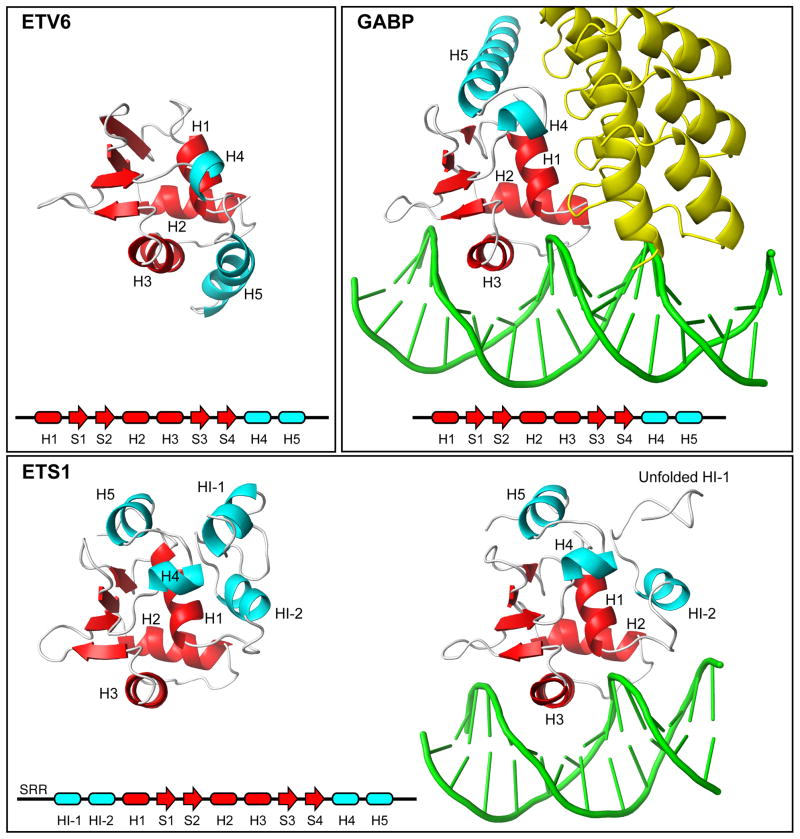
Fig. 7. Appended helices provide specificity to ETS factors.
Shown are the ETS domains (red) and appended helices (cyan) present in free ETV6, the complex of GABPA with GABPB (yellow) and DNA (1AWC.pdb), and ETS1 in its free (1R36.pdb) and DNA-bound (1MDM.pdb) forms. A comparison of these structures shows that the CID helix H5 sterically blocks the DNA-binding interface of ETV6. In contrast, ETS1 contains a distal inhibitory module formed by helices flanking the ETS domain, and helix HI-1 unfolds in an allosteric response to DNA-binding. Multi-site phosphorylation of an unstructured Serine Rich Region (SRR) stabilizes the inhibitory module and thereby increases auto-inhibition.18 In the case of GABP, helix H5 contributes to an interface with the ankyrin repeats of GABPB, and the presence of GABPB indirectly enhances the affinity of GABPA for DNA.42