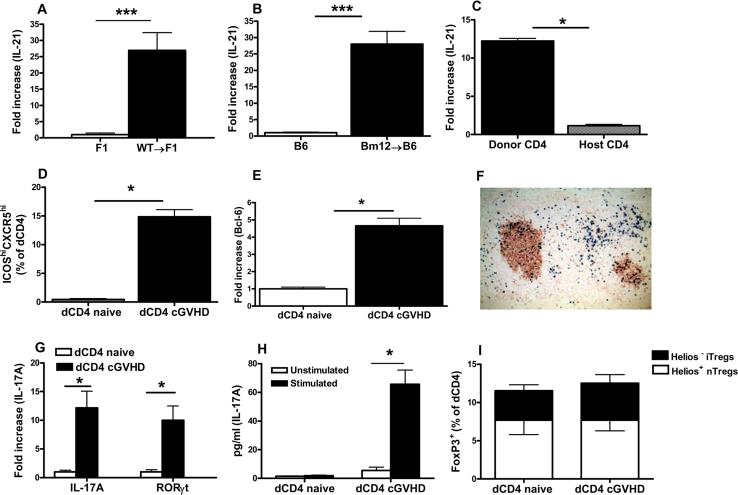
Figure 1. Donor CD4+ T cells produce IL-21 and display TFH, Th17 and Treg cell phenotypes.
A-B. P→F1 cGVHD (A) and Bm12→B6 cGVHD (B) were induced as described in Materials and Methods. Splenocytes were analyzed after 14 days for IL-21 mRNA expression by RT-PCR. Results were normalized to 18S RNA. C. Two weeks after P→F1 cGVHD induction, donor and host CD4+ T cells were isolated from pooled mice as described in Materials and Methods. RT-PCR for IL-21 was performed. D. Mean percentage of donor TFH cells detected by flow cytometry as ICOShiCXCR5hiCD4+H2-Kd- T cells in P→F1 cGVHD mice at 2 weeks after induction. E. Naïve donor CD4+ T cells prior to transfer and engrafted donor CD4+ T cells from cGVHD mice were isolated as described in Materials and Methods and RT-PCR for Bcl-6 was performed. F. P→F1 cGVHD was induced with GFP expressing WT donor cells. Immunohistochemistry staining of spleen section for PNA+ GCs (brown) and donor GFP+ cells (blue) was performed at 10 days after disease induction. G. mRNA expression for IL-17A and RORγt was assessed ex vivo from donor CD4+ T cells purified on day 14 after cGVHD induction. Purified, naive CD4+ T cells prior to transfer were used as controls. H. Purified donor CD4+ T cells were stimulated with anti-CD3, anti-CD28 and IL-23 for 5 days then restimulated with PMA/Ionomycin. Supernatants were tested for IL-17 expression by ELISA. I. Mean percentage of donor Treg cells identified by flow cytometry as FoxP3+ cells among uninjected, naïve donor CD4 cells and engrafted donor CD4+ cells at day 14. Helios staining was used to distinguish FoxP3+Helios+ nTregs and FoxP3+Helios– iTregs. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05. ***=p<0.001