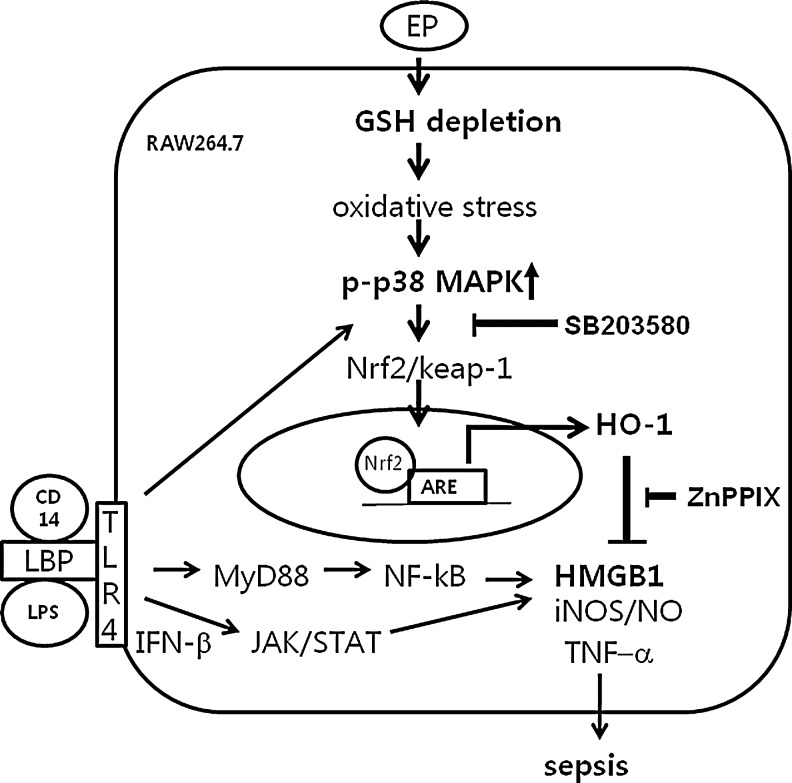
FIG. 9.
Possible mechanism by which EP reduces HMGB1 in sepsis. EP depletes intracellular GSH levels that change redox states of the cell, which, in turn, stimulates p38 MAPK. The activation of p38 MAPK by phosphorylation triggers to activate Nrf2, which dissociates from keap1 and then moves into the nucleus, where it binds to ARE binding sites to induce the HO-1 gene. Thus, SB203580, p38 inhibitor, inhibits EP-mediated HO-1 induction. On the other hand, LPS binds to LPS binding protein (LBP) along with CD14, a recognition molecule for LPS, which activates toll-like receptor 4 (TLR4). The activated TLR4 stimulates p38 MAPK, which then induces HO-1. However, LPS activates NF-κB through MyD88-dependent signal pathways, and IFN-β, generated by LPS through TRIF-dependent signal pathways, activates the JAK/STAT signal pathway to induce inflammatory gene expression, such as iNOS, TNF-α, IL-1β, and release of HMGB1. The induction of HO-1 by EP inhibits these inflammatory cytokines and release of HMGB1 and NO production in LPS-activated RAW 264.7 cells. ZnPPIX, a HO-1 inhibitor, reverses the anti-inflammatory effect of EP (data not shown). Administration of EP also inhibits iNOS expression and circulating TNF-α, IL-1β, and HMGB1 in CLP-induced septic mice, which are dependent on HO-1 induction via activation of p38 MAPK. The schema describes possible signal pathways by which EP activates HO-1 induction in RAW 264.7 cells. IL-1, interleukin-1; NF-κB, nuclear factor kappa B; STAT1, signal transducer and activator of transcription 1; TNF, tumor necrosis factor.