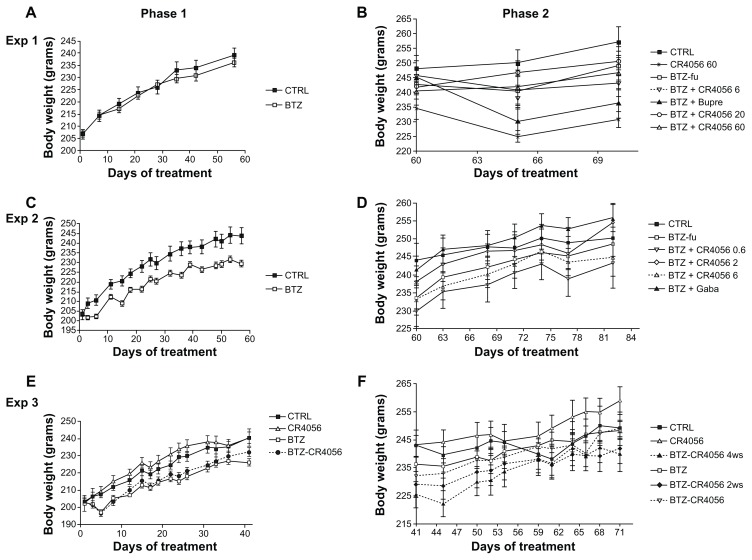
Figure 2.
Body weight changes along the study period: Experiment (Exp) 1, phase (A) 1 and (B) 2; in Exp 1, bortezomib (BTZ)-treated animals do not show any significant difference in weight gain with respect to the control (CTRL) at the end of the 8-week treatment. Exp 2, phase (C) 1 and (D) 2; Exp 3, phase (E) 1 and (F) 2; in Exps 2 and 3, BTZ significantly affected body weight growth (C and E) (P < 0.01); CR4056 when coadministered with BTZ neither worsened nor improved the toxicity induced by BTZ (E). No significant changes were observed in each group between the values measured during phase 2 (B, D, and F).
Note: Data presented as mean plus or minus standard deviation.
Abbreviations: BTZ-fu, bortezomib-fake-manipulated but untreated; Bupre, buprenorphine; CR4056 0.6, CR4056 at 0.6 mg/kg/day; CR4056 2, CR4056 at 2 mg/kg/day; CR4056 20, CR4056 at 20 mg/kg/day; CR4056 6, CR4056 at 6 mg/kg/day; CR4056 60, CR4056 at 60 mg/kg/day; Gaba, gabapentin; ws, weeks.