Abstract
A domestic shorthair cat was presented for lethargy and ataxia. Clinical findings included an abdominal mass, lumbosacral pain, ataxia. Aspirates from the liver and lymph nodes revealed intracellular, negative-staining rods. Treatment for presumptive mycobacterium infection was unsuccessful and the cat was euthanized. Disseminated Mycobacterium avium was confirmed on culture.
A 7-year-old, female, spayed domestic shorthair was referred to the Ontario Veterinary College for moderate weight loss (1.5 kg) and difficulty in walking. Onset of clinical signs coincided with the owners moving to a new house 2 mo previously. The cat was allowed outside under owner supervision but was not allowed to roam. Routine vaccinations were current. No previous therapy was administered.
On physical examination, the cat was in thin body condition (5.0 kg), with generalized muscle wasting and pyrexia (39.9°C). Hydration was judged to be normal. An approximately 4-cm, freely movable, mid-abdominal mass was palpable. Back pain was noted at the lumbosacral junction. Neurological examination revealed hind limb ataxia with normal spinal reflexes. No abnormalities were appreciated in the front limbs. Proprioception was difficult to evaluate due to the cat's weak condition but appeared decreased in the hind limbs. A spinal lesion between the levels of the 6th cervical and 2nd thoracic vertebrae (C6-T2) was suspected to be the cause of the hind limb ataxia and a second spinal lesion was thought to account for the lumbosacral pain. The cat was admitted to the intensive care unit and IV fluids (Plasmalyte-148; Baxter Corporation, Toronto, Ontario) were administered, 14 mL/h, to maintain hydration. Oxymorphone (Numorphan; DuPont Pharma, Mississauga, Ontario), 0.05 mg/kg body weight (BW), IV, PRN, was administered to control pain. Differential diagnoses considered to account for the anorexia, weight loss, ataxia and back pain included neoplasia (lymphosarcoma, fibrosarcoma, meningioma, osteosarcoma, multiple myeloma), sepsis, feline leukemia virus (FeLV), feline immunodeficiency virus (FIV), feline infectious peritonitis (FIP), osteomyelitis, discospondylitis, meningomyelitis, and ehrlichiosis.
Initial diagnostic tests included a complete blood cell (CBC) count, serum biochemical analysis, prothrombin time (PT), partial thromboplastin time (PTT), thoracic radiographs, and abdominal ultrasonography. Urine could not be obtained for a urinalysis. The CBC count showed a low-normal red cell mass (0.25 L/L; reference range, 0.24 to 0.45 L/L), moderate thrombocytosis (780 × 109/L; reference range, 150 to 480 × 109/L), and mild hypoproteinemia (57 g/L; reference range, 60 to 80 g/L). A mild neutrophilia (15.16 × 109/L; reference range, 2.5 to 12.5 × 109/L), moderate lymphopenia (0.65 × 109/L; reference range, 1.5 to 7.0 × 109/L), and a rubricytosis (0.16 × 109/L; reference range, 0.0 × 109/L) were also reported. Abnormalities on the serum biochemical analysis included mild hypocalcemia (1.83 mmol/L; reference range, 2.17 to 2.86 mmol/L), hypoalbuminemia (18 g/L; reference range, 23 to 35 g/L), low urea (4.0 mmol/L; reference range, 4.9 to 11.5 mmol/L), elevated alkaline phosphatase (83 U/L; reference range, 11 to 67 U/L), and elevated amylase (1646 U/L; reference range, 600 to 1300 U/L). The PT and PTT were within normal reference ranges. The antemortem FeLV status of the cat was not determined.
Thoracic radiographs demonstrated diffuse, multifocal, 1 to 2 mm opacities in the lungs. No bony spinal abnormalities were identified. Abdominal ultrasonography revealed several enlarged mesenteric lymph nodes. The largest mesenteric lymph node measured 2 cm × 5 cm and was likely the abdominal mass noted on palpation. In addition, generalized splenomegaly was present and the liver was enlarged and diffusely hyperechoic.
Ultrasonograph-guided aspirates of the liver and enlarged mesenteric lymph nodes were performed. Cytological examination of the liver revealed diffuse hepatocytic vacuolation, interpreted as hepatic lipidosis. There were individual macrophages distended with intracytoplasmic, negative staining, uniform slender rods. A lymph node aspirate contained exclusively macrophages containing a similar population of long intracellular rods, as seen in the liver (Figure 1). Based on the cytologic observations, disseminated mycobacteriosis was suspected.
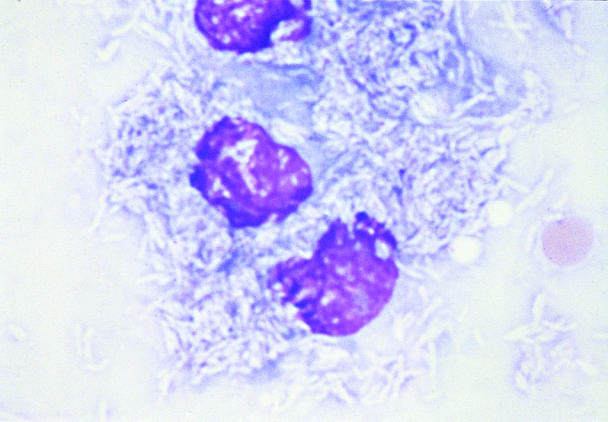
Figure 1. Fine-needle aspirate of mesenteric lymph node. Single population of large macrophages with intracytoplasmic, long, slender, negative-staining, rod-shaped organisms, consistent with mycobacteria; Wright's stain; bar = 1 μm.
Treatment with enrofloxacin (Baytril; Bayer Animal Health, Etobicoke, Ontario), 5.0 mg/kg BW, IV, q12h, and azithromycin (Zithromax; Pfizer Canada, Kirkland, Quebec), 5.0 mg/kg BW, PO, q24h, was initiated. The cat began to have partial complex seizures 6 h after the therapy was started. Due to the declining clinical condition of the cat and the poor prognosis because of the systemic mycobacteriosis, the owners elected for euthanasia.
Postmortem examination revealed enlarged mesenteric and submandibular lymph nodes, hepatosplenomegaly, and diffuse thickening of the ileum. The lungs were firm with generalized, tan miliary foci in all lobes. Histologic examination of mesenteric lymph nodes showed granulomatous, septic lymphadenitis with acid-fast, rod-shaped intracellular bacteria. Similarly, the wall of the ileum contained macrophages containing large numbers of acid-fast rods. Hepatocytes contained lipid vacuoles and numerous hepatic macrophages contained acid-fast organisms. Similar organisms were seen in macrophages from the spleen and bone marrow (Figure 2). No organisms were identified in the neural tissue examined. The pathologic diagnosis was multifocal granulomatous disease, consistent with mycobacteriosis. Mycobacterium avium was confirmed on culture several months later (Canadian Food Inspection Agency, Neapean, Ontario).
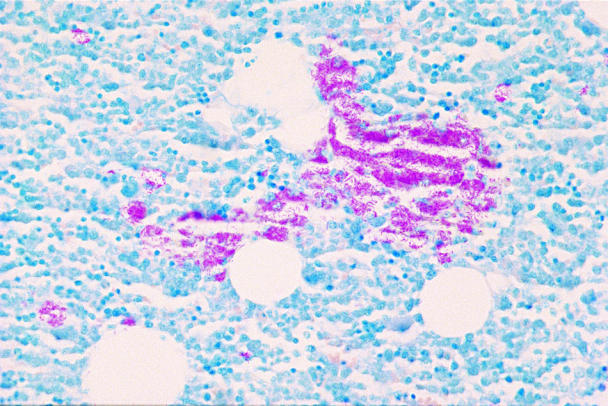
Figure 2. Tissue section of bone marrow. A confluent sheet of stained, fine, rod-shaped organisms is evident interspersed among the hematopoietic cells; Ziehl-Neelsen; bar = 2 μm.
Mycobacterium avium infections have been described in a number of species, including dogs, cats, primates, swine, cattle, sheep, horses, and humans (1,2,3,4,5,6,7,8,9). Descriptions of disseminated infections of M. avium in cats are rare, because cats are naturally resistant to the organism (1,2). An exception to this appears to be the Siamese breed, which is over-represented in reports of M. avium infection and infection with other intracellular organisms (3). Mycobacterium avium belongs to the M. avium-intracellulare complex (MAC), which is a group of slow-growing, atypical mycobacteria (3,4). The complex consists of 28 serovars; 1 through 6 and 8 through 11 are considered to be M. avium (3). Most cases of mycobacteriosis in dogs, cats, and horses are due to serotypes 1, 2, or 4 (4). Mycobacterium avium is a ubiquitous, saprophytic, acid-fast, aerobic, nonspore- forming bacillus that is widely distributed in the environment, especially in water and soil (3,4). Pigs and birds are very susceptible to M. avium infections and may serve as reservoir hosts (4), although the organism may remain viable in the soil for up to 4 y (3,4). Although M. avium is considered an opportunistic organism, it is the most likely of the MAC organisms to produce bacteremia and disseminated disease (3).
Mycobacterium avium is important, because the granulomatous lesions it produces are indistinguishable from the tubercular lesions of M. tuberculosis and M. bovis (3). Mycobacteria produce a cell-mediated, delayed-type hypersensitivity response, characterized by granulomatous inflammation (4). The progression of disease depends on the ability of the macrophages to inhibit intracellular growth of the organisms (4). A competent cell-mediated immune response is more likely to result in elimination of the organism than is an antibody-mediated immune response (3,5).
Clinical signs are typically organ-specific, but nonspecific generalized symptoms, such as weight loss, anorexia, and fever, are common, as was seen in this case (5). Cutaneous lesions with M. avium infection are only rarely reported, but are typically displayed in cats infected with M. lepraemurium (6,7). Mycobacteria often gain entry to the body through either the respiratory tract, the gastrointestinal tract, or the skin, where they are phagocytized by local tissue macrophages and then disseminated to adjacent tissues (8). It is possible that in this case, the ileum was the site of entry, because it was the only part of the gastrointestinal tract affected. Routine clinical laboratory findings of M. avium infection are nonspecific and include anemia, neutrophilic leukocytosis, and hyperglobulinemia (3,6). Infrequently, intracellular organisms can be seen in neutrophils and monocytes on blood smears. A blood film from this cat was reviewed, and infrequent intracellular, unstained rods were identified within circulating monocytes. A presumptive diagnosis of M. avium infection can usually be made following acid-fast staining of affected tissue, although occasionally staining will be negative in affected animals (6). Unlike other mycobacteria, M. avium tends to be present in high numbers within cells (6,11). Definitive diagnosis requires culture of the organism, which can take several weeks in the case of M. avium (3,10). Due to the progressive nature of the disease, treatment should be initiated upon finding acid-fast bacilli and not withheld pending culture results. Recently, polymerase chain reaction has shown promise for the rapid detection of mycobacteria in both humans and cats, although presently its use is limited to the identification of M. tuberculosis (3,12).
In humans, infections with MAC organisms are often associated with immunodeficiency or immunosuppression (3,9). Although one would expect FeLV- or FIV-positive cats to be predisposed to mycobacterial infections, a significant association has not been found (3,9). In several reports of feline mycobacteriosis, the cats were found to be FeLV negative at the time of diagnosis (1,2,6,10). Feline immunodeficiency virus status was not assessed in the cats reported previously or in this case.
In the case presented here, a retroviral infection cannot be entirely excluded as a predisposing cause of the M. avium infection. Postmortem bone marrow was assessed for the presence of FeLV using immunohistochemical analysis (Prairie Diagnostic Services, Saskatoon, Saskatchewan). Weak cross reactivity was seen between the FeLV reagent and an M. bovis-positive control, so it was unclear if there was mycobacterium antigen being recognized by using the FeLV reagent. The results of the immunohistochemical analysis did not support the presence of an FeLV infection. Other infectious agents or immune dysfunction may also have predisposed the cat to opportunistic mycobacterial infection.
There are rare reports of cats with M. avium infection being successfully treated using combination antibiotic therapy including rifampin, enrofloxacin, and clarithromycin (3,6). Treatment times can range from 6 wk to 6–8 mo (3). Although an initial response to treatment can be achieved, resulting in remission of clinical signs, relapses are common and have been reported up to 2 y following treatment (3). Consideration must also be given to the possible zoonotic potential of affected cats prior to attempting treatment.
Human cases of M. avium infection are becoming increasingly common (9). It has generally been assumed that only immunosuppressed individuals or those with existing pulmonary disease are at risk of contracting M. avium infection through direct acquisition of the organism from contaminated environments (3,9). However, there are increasing reports of M. avium infection in humans who do not have predisposing risk factors (9). Although there are no confirmed reports of transmission of M. avium between animals and humans, reasonable precautions should be taken by anyone handling affected animals (3). In particular, special attention should be paid to the appropriate handling and disposal of used needles to prevent accidental inoculation of the organism.
Mycobacterium avium infections are only rarely reported in domestic animals and are usually opportunistic infections. Unfortunately treatment failures and relapses are common in dogs and cats infected with M. avium. With the increasing prevalence of human infections with MAC organisms, appropriate handling of known or suspected cases to prevent accidental zoonotic transmission is prudent. CVJ
Footnotes
Address correspondence to Dr. Maureen Barry.
Reprints will not be available from the authors.
References
- 1.Drolet R. Disseminated tuberculosis caused by Mycobacterium avium in a cat. J Am Vet Med Assoc 1986;189:1336–1337. [PubMed]
- 2.Buergelt CD, Fowler JL, Wright PJ. Disseminated avian tuberculosis in a cat. Calif Vet 1982;10:13–15.
- 3.Greene CE, Gunn-Moore DA. Mycobacterial infections. In: Green CE, ed. Infectious Diseases of the Dog and Cat. 2nd ed. Philadelphia: WB Saunders, 1990:313–315.
- 4.Zeiss CJ, Jardine J, Huchzermeyer H. A case of disseminated tuberculosis in a dog caused by Mycobacterium avium-intracellulare. J Am Anim Hosp Assoc 1994;30:419–424.
- 5.Miller MA, Greene CE, Brix AE. Disseminated Mycobacterium avium-intracellulare complex infection in a miniature schnauzer. J Am Anim Hosp Assoc 1995;31:213–216. [DOI] [PubMed]
- 6.Lemarie SL. Mycobacterial dermatitis. Vet Clin North Am Small Anim Pract 1999;29:1291–1301. [DOI] [PubMed]
- 7.Roccabianca P, Caniatti M, Scanziani E, Penati V. Feline leprosy: spontaneous remission in a cat. J Am Anim Hosp Assoc 1996;32: 189–193. [DOI] [PubMed]
- 8.Shackelford CC, Reed WM. Disseminated Mycobacterium avium infection in a dog. J Vet Diagn Invest 1989;1:273–275. [DOI] [PubMed]
- 9.Iseman MD. Mycobacterium avium complex and the normal host: the other side of the coin. N Eng J Med 1989;321:896–898. [DOI] [PubMed]
- 10.Morfitt DC, Matthews JA, Theon CO, Kluge JP. Disseminated Mycobacterium avium serotype 1 infection in a seven-month-old cat. J Vet Diagn Invest 1989;1:354–356. [DOI] [PubMed]
- 11.Clercx C, Coignoul F, Jakovljevic S, et al. Tuberculosis in dogs: a case report and review of the literature. J Am Anim Hosp Assoc 1992;28:207–211.
- 12.Barnes PF, Barrows SA. Tuberculosis in the 1990s. Ann Intern Med 1993;119:400–410. [DOI] [PubMed]


