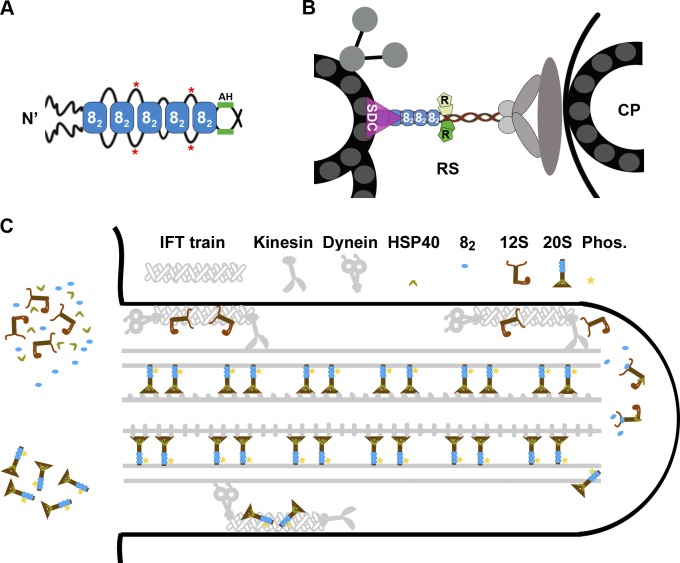
Figure 10.
Models depicting the effects of LC8 on the RS complex. (A) Multiple LC8 dimers (blue rectangles, 82) associate with a dimeric RSP3 (black lines) at the region N terminal (N’) upstream of the AH. The sequences between the LC8-binding sites may loop out of the stack and become phosphorylated (red asterisks). (B) The dimeric RSP3 N terminus and the stack of LC8 form the basal part of the RS. Phosphorylation near the LC8-binding sites may alter local conformation, promoting the interactions of the nearby AH with homodimeric RIIa domains (R) and the interaction of the axonemal binding region with the spoke-docking complex (SDC), possibly the CSC, or a FAP206-containing complex. Only a fraction of the 9 + 2 axoneme and relevant RSPs are illustrated. CP, central pair. (C) The two-stage assembly process of the RS complex in the cell body (left) and flagellum (right). LC8 dimer and HSP40 dimer do not join the RS until the L-shaped 12S RS precursors are transported into flagella by kinesin-driven IFT. Association of HSP40 with the 12S RS transforms the spokehead, whereas binding of LC8 to the N-terminal region of RSP3 in the 12S RS triggers formation of the stalk base, phosphorylation (Phos.) of RSP3 (asterisks), and docking of the T-shaped 20S RS to the axoneme. The unbound 20S RSs are delivered back to the cell body by dynein-driven IFT. The unknown transport mechanisms of LC8 and HSP40 are not depicted.